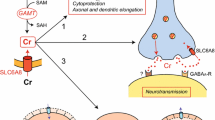
Abstract
Purpose
Cyclocreatine, a creatine analog, is a candidate drug for treating patients with cerebral creatine deficiency syndromes (CCDSs) caused by creatine transporter (CRT, SLC6A8) deficiency, which reduces brain creatine level. The purpose of this study was to clarify the characteristics of cyclocreatine transport in HEK293 cells, which highly express endogenous CRT, in hCMEC/D3 cells, a human blood-brain barrier (BBB) model, and in CCDSs patient-derived fibroblasts with CRT mutations.
Methods
Cells were incubated at 37°C with [14C]cyclocreatine (9 μM) and [14C]creatine (9 μM) for specified periods of times in the presence or absence of inhibitors, while the siRNAs were transfected by lipofection. Protein expression and mRNA expression were quantified using targeted proteomics and quantitative PCR, respectively.
Results
[14C]Cyclocreatine was taken up by HEK293 cells in a time-dependent manner, while exhibiting saturable kinetics. The inhibition and siRNA knockdown studies demonstrated that the uptake of [14C]cyclocreatine by both HEK293 and hCMEC/D3 cells was mediated predominantly by CRT as well as [14C]creatine. In addition, uptake of [14C]cyclocreatine and [14C]creatine by the CCDSs patient-derived fibroblasts was found to be largely reduced.
Conclusion
The present study suggests that cyclocreatine is a CRT substrate, where CRT is the predominant contributor to influx of cyclocreatine into the brain at the BBB. Our findings provide vital insights for the purposes of treating CCDSs patients using cyclocreatine.






Similar content being viewed by others
Abbreviations
- ADP:
-
Adenosine diphosphate
- AGAT:
-
L-Arginine: glycine amidinotransferase
- ATP:
-
Adenosine triphosphate
- BAP31:
-
B cell receptor-associated protein
- BBB:
-
Blood-brain barrier
- bFGF:
-
Basic fibroblast growth factor
- CaMK2a:
-
Ca2+/calmodulin-dependent protein kinase a
- CCDSs:
-
Cerebral creatine deficiency syndromes
- CRT:
-
Creatine transporter
- DMEM:
-
Dulbecco’s modified Eagle medium
- FBS:
-
Fetal bovine serum
- GAMT:
-
Guanidinoacetate methyltransferase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GC:
-
Graphite carbon
- GPA:
-
β-guanidinopropionic acid
- HBSS:
-
Hank’s balanced salt solution
- hCMEC:
-
Human cerebral microvascular endothelial cell
- HEPES:
-
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- MRM:
-
Multiple reaction monitoring
- PTS:
-
Phase-transfer surfactant
- SDB:
-
Styrene-divinylbenzene
- SLC6A8:
-
Solute carrier family 6 member 8
References
Rosenberg EH, Almeida LS, Kleefstra T, deGrauw RS, Yntema HG, Bahi N, et al. High prevalence of SLC6A8 deficiency in X-linked mental retardation. Am J Hum Genet. 2004;75:97–105.
Salomons GS, van Dooren SJ, Verhoeven NM, Marsden D, Schwartz C, Cecil KM, et al. X-linked creatine transporter defect: an overview. J Inherit Metab Dis. 2003;26:309–18.
deGrauw TJ, Cecil KM, Byars AW, Salomons GS, Ball WS, Jakobs C. The clinical syndrome of creatine transporter deficiency. Mol Cell Biochem. 2003;244:45–8.
Almeida LS, Verhoeven NM, Roos B, Valongo C, Cardoso ML, Vilarinho L, et al. Creatine and guanidinoacetate: diagnostic markers for inborn errors in creatine biosynthesis and transport. Mol Genet Metab. 2004;82:214–9.
Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–213.
Mercimek-Mahmutoglu S, Stoeckler-Ipsiroglu S, Adami A, Appleton R, Araujo HC, Duran M, et al. GAMT deficiency: features, treatment, and outcome in an inborn error of creatine synthesis. Neurology. 2006;67:480–4.
Battini R, Leuzzi V, Carducci C, Tosetti M, Bianchi MC, Item CB, et al. Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree. Mol Genet Metab. 2002;77:326–31.
Ohtsuki S, Tachikawa M, Takanaga H, Shimizu H, Watanabe M, Hosoya K, et al. The blood-brain barrier creatine transporter is a major pathway for supplying creatine to the brain. J Cereb Blood Flow Metab. 2002;22:1327–35.
Stockler-Ipsiroglu S, van Karnebeek C, Longo N, Korenke GC, Mercimek-Mahmutoglu S, Marquart I, et al. Guanidinoacetate methyltransferase (GAMT) deficiency: outcomes in 48 individuals and recommendations for diagnosis, treatment and monitoring. Mol Genet Metab. 2014;111:16–25.
Edvardson S, Korman SH, Livne A, Shaag A, Saada A, Nalbandian R, et al. L-arginine:glycine amidinotransferase (AGAT) deficiency: clinical presentation and response to treatment in two patients with a novel mutation. Mol Genet Metab. 2010;101:228–32.
Schulze A. Creatine deficiency syndromes. Mol Cell Biochem. 2003;244:143–50.
Poo-Arguelles P, Arias A, Vilaseca MA, Ribes A, Artuch R, Sans-Fito A, et al. X-linked creatine transporter deficiency in two patients with severe mental retardation and autism. J Inherit Metab Dis. 2006;29:220–3.
Cecil KM, Salomons GS, Ball WS Jr, Wong B, Chuck G, Verhoeven NM, et al. Irreversible brain creatine deficiency with elevated serum and urine creatine: a creatine transporter defect? Ann Neurol. 2001;49:401–4.
Rudnick G, Kramer R, Blakely RD, Murphy DL, Verrey F. The SLC6 transporters: perspectives on structure, functions, regulation, and models for transporter dysfunction. Pflugers Arch. 2014;466:25–42.
van de Kamp JM, Mancini GM, Salomons GS. X-linked creatine transporter deficiency: clinical aspects and pathophysiology. J Inherit Metab Dis. 2014;37:715–33.
Uemura T, Ito S, Ohta Y, Tachikawa M, Wada T, Terasaki T, et al. Abnormal N-glycosylation of a novel missense Creatine transporter mutant, G561R, Associated with Cerebral Creatine Deficiency Syndromes Alters Transporter Activity and Localization. Biol Pharm Bull. 2017;40:49–55.
Rosenberg EH, Martinez Munoz C, Betsalel OT, van Dooren SJ, Fernandez M, Jakobs C, et al. Functional characterization of missense variants in the creatine transporter gene (SLC6A8): improved diagnostic application. Hum Mutat. 2007;28:890–6.
Betsalel OT, Pop A, Rosenberg EH, Fernandez-Ojeda M, Jakobs C, Salomons GS. Detection of variants in SLC6A8 and functional analysis of unclassified missense variants. Mol Genet Metab. 2012;105:596–601.
Mak CS, Waldvogel HJ, Dodd JR, Gilbert RT, Lowe MT, Birch NP, et al. Immunohistochemical localisation of the creatine transporter in the rat brain. Neuroscience. 2009;163:571–85.
Dodd JR, Birch NP, Waldvogel HJ, Christie DL. Functional and immunocytochemical characterization of the creatine transporter in rat hippocampal neurons. J Neurochem. 2010;115:684–93.
Skelton MR, Schaefer TL, Graham DL, Degrauw TJ, Clark JF, Williams MT, et al. Creatine transporter (CrT; Slc6a8) knockout mice as a model of human CrT deficiency. PLoS One. 2011;6:e16187.
Baroncelli L, Molinaro A, Cacciante F, Alessandri MG, Napoli D, Putignano E, et al. A mouse model for creatine transporter deficiency reveals early onset cognitive impairment and neuropathology associated with brain aging. Hum Mol Genet. 2016;25:4186–200.
Baroncelli L, Alessandri MG, Tola J, Putignano E, Migliore M, Amendola E, et al. A novel mouse model of creatine transporter deficiency. F1000Res. 2014;3:228.
Woznicki DT, Walker JB. Formation of a supplemental long time-constant reservoir of high energy phosphate by brain in vivo and in vitro and its reversible depletion by potassium depolarization. J Neurochem. 1979;33:75–80.
Kurosawa Y, Degrauw TJ, Lindquist DM, Blanco VM, Pyne-Geithman GJ, Daikoku T, et al. Cyclocreatine treatment improves cognition in mice with creatine transporter deficiency. J Clin Invest. 2012;122:2837–46.
Halestrap AP. Monocarboxylic acid transport. Compr Physiol. 2013;3:1611–43.
Osaka H, Takagi A, Tsuyusaki Y, Wada T, Iai M, Yamashita S, et al. Contiguous deletion of SLC6A8 and BAP31 in a patient with severe dystonia and sensorineural deafness. Mol Genet Metab. 2012;106:43–7.
Kato H, Miyake F, Shimbo H, Ohya M, Sugawara H, Aida N, et al. Urine screening for patients with developmental disabilities detected a patient with creatine transporter deficiency due to a novel missense mutation in SLC6A8. Brain and Development. 2014;36:630–3.
Masuda T, Hoshiyama T, Uemura T, Hirayama-Kurogi M, Ogata S, Furukawa A, et al. Large-scale quantitative comparison of plasma Transmembrane proteins between two human blood-brain barrier model cell lines, hCMEC/D3 and HBMEC/cibeta. Mol Pharm. 2019;16:2162–71.
Masuda T, Tomita M, Ishihama Y. Phase transfer surfactant-aided trypsin digestion for membrane proteome analysis. J Proteome Res. 2008;7:731–40.
Nakamura K, Hirayama-Kurogi M, Ito S, Kuno T, Yoneyama T, Obuchi W, et al. Large-scale multiplex absolute protein quantification of drug-metabolizing enzymes and transporters in human intestine, liver, and kidney microsomes by SWATH-MS: comparison with MRM/SRM and HR-MRM/PRM. Proteomics. 2016;16:2106–17.
Pis-Diez R, Parajón-Costa BS, Franca CA, Piro OE, Castellano EE, González-Baró AC. Cyclocreatine, an anticancer and neuroprotective agent. Spectroscopic, structural and theoretical study. J Mol Struct. 2010;975:303–9.
Dai W, Vinnakota S, Qian X, Kunze DL, Sarkar HK. Molecular characterization of the human CRT-1 creatine transporter expressed in Xenopus oocytes. Arch Biochem Biophys. 1999;361:75–84.
Marescau B, De Deyn P, Wiechert P, Van Gorp L, Lowenthal A. Comparative study of guanidino compounds in serum and brain of mouse, rat, rabbit, and man. J Neurochem. 1986;46:717–20.
Harris RC, Soderlund K, Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin Sci (Lond). 1992;83:367–74.
Marescau B, Deshmukh DR, Kockx M, Possemiers I, Qureshi IA, Wiechert P, et al. Guanidino compounds in serum, urine, liver, kidney, and brain of man and some ureotelic animals. Metabolism. 1992;41:526–32.
Wang AQ, Hughes E, Huang W, Kerns EH, Xu X. Quantification of cyclocreatine in mouse and rat plasma using hydrophilic-interaction ultra-performance liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2017;145:629–33.
Enrico A, Patrizia G, Luisa P, Alessandro P, Gianluigi L, Carlo G, et al. Electrophysiology and biochemical analysis of cyclocreatine uptake and effect in hippocampal slices. J Integr Neurosci. 2013;12:285–97.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported in part by JSPS KAKENHI Grant Number JP26293035 and JP18H02590, and by AMED under Grant Number JP19ek0109396. Ohtsuki S is a full professor at Kumamoto University and is also a director of Proteomedix Frontiers. The other authors declare no competing interests.
Author information
Authors and Affiliations
Contributions
All authors, Uemura T, Ito S, Masuda T, Shimbo H, Goto T, Osaka H, Wada T, Couraud PO, Ohtsuki S, contributed to study design and manuscript revision. Uemura T conducted the experiments and performed data analysis. Uemura T, Ito S. and Ohtsuki S wrote the manuscript. All the authors have provided their final approval for submission of this manuscript.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uemura, T., Ito, S., Masuda, T. et al. Cyclocreatine Transport by SLC6A8, the Creatine Transporter, in HEK293 Cells, a Human Blood-Brain Barrier Model Cell, and CCDSs Patient-Derived Fibroblasts. Pharm Res 37, 61 (2020). https://doi.org/10.1007/s11095-020-2779-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-2779-0