Abstract
Purpose
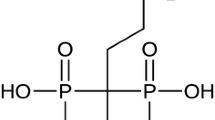
Amifostine (AMF), a radioprotectant, is FDA-approved for intravenous administration in cancer patients receiving radiation therapy (XRT). Unfortunately, it remains clinically underutilized due to adverse side effects. The purpose of this study is to define the pharmacokinetic profile of an oral AMF formulation potentially capable of reducing side effects and increasing clinical feasibility.
Methods
Calvarial osteoblasts were radiated under three conditions: no drug, AMF, and WR-1065 (active metabolite). Osteogenic potential of cells was measured using alkaline phosphatase staining. Next, rats were given AMF intravenously or directly into the jejunum, and pharmacokinetic profiles were evaluated. Finally, rats were given AMF orally or subcutaneously, and blood samples were analyzed for pharmacokinetics.
Results
WR-1065 preserved osteogenic potential of calvarial osteoblasts after XRT to a greater degree than AMF. Direct jejunal AMF administration incurred a systemic bioavailability of 61.5%. Subcutaneously administrated AMF yielded higher systemic levels, a more rapid peak exposure (0.438 vs. 0.875 h), and greater total systemic exposure of WR-1065 (116,756 vs. 16,874 ng*hr/ml) compared to orally administered AMF.
Conclusions
Orally administered AMF achieves a similar systemic bioavailability and decreased peak plasma level of WR-1065 compared to intravenously administered AMF, suggesting oral AMF formulations maintain radioprotective efficacy without causing onerous side effects, and are clinically feasible.



Similar content being viewed by others
Abbreviations
- %F :
-
Systemic bioavailability
- ALP:
-
Alkaline Phosphatase
- alpha-MEM:
-
Alpha-modified minimal essential medium
- AMF:
-
Amifostine
- AUC :
-
Areas under curve
- Cmax :
-
Maximum concentration
- EC-AMF :
-
Enteric-coated AMF
- HNC :
-
Head and neck cancer
- IV :
-
Intravenous
- LC-MS:
-
Liquid chromatography-mass spectrometry
- MC3T3 cells :
-
Calvarial osteoblasts
- MS:
-
Mass spectrometry
- ODM :
-
Osteogenic differentiation medium
- PBS:
-
Phosphate Buffered Saline
- PK:
-
Pharmacokinetic
- PO:
-
Oral
- SC :
-
Subcutaneous
- UPLC:
-
Ultra performance liquid chromatography
- WR-1065:
-
Active Amifostine metabolite
- WR-1065-EMI :
-
WR-1065 N-ethylmaleimide
- WR-1065-MMI :
-
Modified WR-1065 N-methymaleimide
- XRT:
-
Radiation therapy
References
Cohen EM, Schapira L. Head and Neck Cancer: Statistics. Cancer.net.; 2017 September. Available from: http://www.cancer.net/cancer-types/head-and-neck-cancer/statistics
Moreau MF, Gallois Y, Baslé MF, Chappard D. Gamma irradiation of human bone allografts alters medullary lipids and releases toxic compounds for osteoblast-like cells. Biomaterials. 2000;21(4):369–76.
Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283–8.
Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64(4):379–90.
Monson LA, Nelson NS, Donneys A, Farberg AS, Tchanque-Fossuo CN, Deshpande SS, et al. Amifostine treatment mitigates the damaging effects of radiation on distraction osteogenesis in the murine mandible. Ann Plast Surg. 2016;77(2):164–8.
Donneys A, Nelson NS, Page EE, Deshpande SS, Felice PA, Tchanque-Fossuo CN, et al. Targeting angiogenesis as a therapeutic means to reinforce osteocyte survival and prevent nonunions in the aftermath of radiotherapy. Head Neck. 2015;37(9):1261–7.
Wu HY, Hu ZH, Jin T. Sustained-release microspheres of amifostine for improved radio-protection, patient compliance, and reduced side effects. Drug Deliv. 2016;23(9):3704–11.
Kamran MZ, Ranjan A, Kaur N, Sur S, Tandon V. Radioprotective agents: strategies and translational advances. Med Res Rev. 2016;36(3):461–93.
Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-Spectrum Radioprotector. Oncologist. 2007;12(6):738–47.
Felice PA, Gong B, Ahsan S, Deshpande SS, Nelson NS, Donneys A, et al. Raman spectroscopy delineates radiation-induced injury and partial rescue by amifostine in bone: a murine mandibular model. J Bone Miner Metab. 2015;33(3):279–84.
Tchanque-Fossuo CN, Donneys A, Razdolsky ER, Monson LA, Farberg AS, Deshpande SS, et al. Quantitative histologic evidence of Amifostine-induced Cytoprotection in an irradiated murine model of mandibular distraction osteogenesis. Plast Reconstr Surg. 2012;130(6):1199–207.
Donneys A, Tchanque-Fossuo CN, Blough JT, Nelson NS, Deshpande SS, Buchman SR. Amifostine preserves osteocyte number and osteoid formation in fracture healing following radiotherapy. J Oral Maxillofac Surg. 2014;72(3):559–66.
Page EE, Deshpande SS, Nelson NS, Felice PA, Donneys A, Rodriguez JJ, et al. Prophylactic administration of Amifostine protects vessel thickness in the setting of irradiated bone. J Plast Reconstr Aesthet Surg. 2015;68(1):98–103.
Sarhaddi D, Tchanque-Fossuo CN, Poushanchi B, Donneys A, Deshpande SS, Weiss DM, et al. Amifostine protects vascularity and improves Union in a Model of irradiated mandibular fracture healing. Plast Reconstr Surg. 2013;132(6):1542–9.
Polyatskaya Y, Nelson NS, Rodriguez JJ, Zheutlin AR, Deshpande SS, Felice PA, et al. Prophylactic amifostine prevents a pathologic vascular response in a murine model of expander-based breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69(2):234–40.
Tchanque-Fossuo CN, Gong B, Poushanchi B, Donneys A, Sarhaddi D, Gallagher KK, et al. Raman spectroscopy demonstrates Amifostine induced preservation of bone mineralization patterns in the irradiated murine mandible. Bone. 2013;52(2):712–7.
Felice PA, Ahsan S, Perosky JE, Deshpande SS, Nelson NS, Donneys A, et al. Prophylactic Amifostine preserves the biomechanical properties of irradiated bone in the murine mandible. Plast Reconstr Surg. 2014;133(3):314e–21e.
Tchanque-Fossuo CN, Donneys A, Deshpande SS, Nelson NS, Boguslawski MJ, Gallagher KK, et al. Amifostine remediates the degenerative effects of radiation on the mineralization capacity of the murine mandible. Plast Reconstr Surg. 2012;129(4):646e–55e.
US Food and Drug Administration. Ethyol® (amifostine) for injection. Washington, D.C.
Calabro-Jones PM, Fahey RC, Smoluk GD, Ward JF. Alkaline phosphatase promotes radioprotection and accumulation of WR-1065 in V79-171 cells incubated in medium containing WR-2721. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;47(1):23–7.
Ryan SV, Carrithers SL, Parkinson SJ, Shurk C, Nuss C, Pooler PM. Hypotensive mechanisms of amifostine. J Clin Pharmacol. 1996;36(4):365–73.
Gula A, Ren L, Zhou Z, Lu D, Wang S. Design and evaluation of biodegradable enteric microcapsules of amifostine for oral delivery. Int J Pharm. 2013;453(2):441–7.
Oppenheimer AJ, Rhee ST, Goldstein SA, Buchman SR. Force-induced craniosynostosis in the murine sagittal suture. Plast Reconstr Surg. 2009;124(6):1840–8.
Rickard DJ, Kassem M, Hefferan TE, Sarkar G, Spelsberg TC, Riggs BL. Isolation and characterization of osteoblast precursor cells from human bone marrow. J Bone Miner Res. 2009;11(3):312–24.
Calabro-Jones PM, Aquilera JA, Ward JF, Smoluk GD, Fahey RC. Uptake of WR-2721 derivatives by cells in culture: identification of the transported form of the drug. Cancer Res. 1988;48:3634–40.
Akbulut S, Sevmis S, Karakayali H, Bayraktar N, Unlukaplan M, Oksuz E, et al. Amifostine enhances the antioxidants and hepatoprotective effects of UW and HTK preservation solutions. World J Gastroenterol. 2014;20(34):12292–300.
Santini V, Giles FJ. The potential of amifostine: from cytoprotectant to therapeutic agent. Haematologica. 1999;84(11):1035–42.
Verstappen CC, Postma TJ, Geldof AA, Heimans JJ. Amifostine protects against chemotherapy-induced neurotoxicity: an in vitro investigation. Anticancer Res. 2004;24(4):2337–41.
Jellema AP, Slotman BJ, Muller MJ, Leemans CR, Smeele EL, Hoekman K, et al. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly. Cancer. 2006;107(3):544–53.
Akbulut S, Elbe H, Eric C, Dogan Z, Toprak G, Otan E, et al. Cytoprotective effects of amifostine, ascorbic acid and N-acetylcysteine against methotrexate-induced hepatotoxicity in rats. World J Gastroenterol. 2014;20(29):10158–65.
Mandal TK, Bostanian LA, Graves RA, Chapman SR, Womack I. Development of biodegradable microcapsules as carrier for oral controlled delivery of amifostine. Drug Dev Ind Pharm. 2002;28(3):339–44.
Pamujula S, Kishore V, Rider B, Fermin CD, Graves RA, Agrawal KC, et al Radioprotection in mice following oral delivery of amifostine nanoparticles. Int J Radiat Biol. 2005;81(3):251–7.
Acknowledgments and Disclosures
This work was support by R01 CA 125187 awarded to Steven R. Buchman, MD by the Nation Institutes of Health (NIH).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranganathan, K., Simon, E., Lynn, J. et al. Novel Formulation Strategy to Improve the Feasibility of Amifostine Administration. Pharm Res 35, 99 (2018). https://doi.org/10.1007/s11095-018-2386-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2386-5