Abstract
Purpose
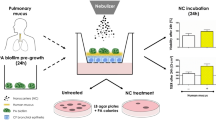
The failure of chronic therapy with antibiotics to clear persistent respiratory infection is the key morbidity and mortality factor for patients with chronic lung diseases, primarily due to the presence of biofilm in the lungs. It is hypothesised that carbon sources, such as mannitol, could stimulate the metabolic activity of persister cells within biofilms and restore their susceptibility to antibiotics. The aims of the current study are to: (1) establish a representative in vitro model of Pseudomonas aeruginosa biofilm lung infection, and (2) investigate the effects of nebulised mannitol on antibiotic efficacy, focusing on ciprofloxacin, in the eradication of biofilm.
Method
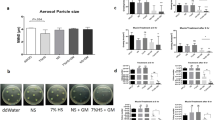
Air interface biofilm was cultured onto Snapwell inserts incorporated into a modified pharmacopeia deposition apparatus, the Anderson Cascade Impactor (ACI). Three different formulations including mannitol only, ciprofloxacin only and combined ciprofloxacin and mannitol were nebulised onto the P. aeruginosa biofilm using the modified ACI. Antibacterial effectiveness was evaluated using colony-forming units counts, biofilm penetration and scanning electron microscopy.
Results
Nebulised mannitol promotes the dispersion of bacteria from the biofilm and demonstrated a synergistic enhancement of the antibacterial efficacy of ciprofloxacin compared to delivery of antibiotic alone.
Conclusions
The combination of ciprofloxacin and mannitol may provide an important new strategy to improve antibiotic therapy for the treatment of chronic lung infections. Furthermore, the development of a representative lung model of bacterial biofilm could potentially be used as a platform for future new antimicrobial pre-clinical screening.







Similar content being viewed by others
Abbreviations
- ACI:
-
Anderson cascade impactor
- ATCC:
-
American type cell culture collection
- BE:
-
Bronchiectasis
- CAMHB:
-
Cation adjusted Mueller-Hinton broth
- Cipro:
-
Ciprofloxacin only
- Cipro-man:
-
Ciprofloxacin and mannitol
- CDC:
-
Centers for disease control
- CF:
-
Cystic fibrosis
- CFU:
-
Colony-forming unit count
- COPD:
-
Chronic obstructive pulmonary disease
- CV:
-
Crystal violet
- DMEM:
-
Dulbecco’s Modified Eagle’s Medium
- DMSO:
-
Dimethyl sulfoxide
- EPS:
-
Extracellular polymeric substances
- EVOM:
-
Epithelial voltohmmeter
- f 1 :
-
Difference factor
- f 2 :
-
Similarity factor
- FBS:
-
Fetal bovine serum
- FPF:
-
Fine particle fraction
- GSD:
-
Geometric standard deviation
- HBSS:
-
Hank’s balanced salt solution
- HPLC:
-
High performance liquid chromatography
- LB:
-
Luria-Bertani
- MMAD:
-
Mass median aerodynamic diameter
- P. aeruginosa :
-
Pseudomonas aeruginosa
- PBS:
-
Phosphate buffered saline
- PCF:
-
Primary ciliary dyskinesia
- RID:
-
Refractive index detector
- RTIs:
-
Respiratory tract infections
- SEM:
-
Scanning electron microscope
- TEER:
-
Transepithelial electrical resistance
- USP:
-
United States Pharmacopoeia
References
Organization WH. WHO: The top 10 causes of death. 2014 [Available from: http://www.who.int/mediacentre/factsheets/fs310/en/.
Hassett DJ, Cuppoletti J, Trapnell B, Lymar SV, Rowe JJ, Yoon SS, et al. Anaerobic metabolism and quorum sensing by Pseudomonas Aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv Drug Deliv Rev. 2002;54(11):1425–43.
McShane PJ, Naureckas ET, Tino G, Strek ME. Non–cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. 2013;188(6):647–56.
Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359(22):2355–65.
Bjarnsholt T, Jensen PØ, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, et al. Pseudomonas Aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol. 2009;44(6):547–58.
Clancy J, Dupont L, Konstan M, Billings J, Fustik S, Goss C, et al. Phase II studies of nebulised Arikace in CF patients with Pseudomonas Aeruginosa infection. Thorax. 2013; 68(9): 818-825. https://doi.org/10.1136/thoraxjnl-2012-202230.
Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ. Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas Aeruginosa. Am J Respir Crit Care Med. 2011;183(11):1510–6.
Serisier DJ, Bilton D, De Soyza A, Thompson PJ, Kolbe J, Greville HW, et al. Inhaled, dual release liposomal ciprofloxacin in non-cystic fibrosis bronchiectasis (ORBIT-2): a randomised, double-blind, placebo-controlled trial. Thorax. 2013;68(9):812–7.
Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, et al. Effects of reduced mucus oxygen concentration in airway pseudomonas infections of cystic fibrosis patients. J Clin Invest. 2002;109(3):317–25.
Sousa AM, Pereira MO. Pseudomonas Aeruginosa diversification during infection development in cystic fibrosis lungs—a review. Pathogens. 2014;3(3):680–703.
Lebeaux D, Ghigo J-M, Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiol Mol Biol Rev. 2014;78(3):510–43.
Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011;473(7346):216.
Barraud N, Buson A, Jarolimek W, Rice SA. Mannitol enhances antibiotic sensitivity of persister bacteria in Pseudomonas Aeruginosa biofilms. PLoS One. 2013;8(12):e84220.
Lebeaux D, Chauhan A, Rendueles O, Beloin C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens. 2013;2(2):288–356.
Santopolo L, Marchi E, Frediani L, Decorosi F, Viti C, Giovannetti L. A novel approach combining the Calgary biofilm device and phenotype MicroArray for the characterization of the chemical sensitivity of bacterial biofilms. Biofouling. 2012;28(9):1023–32.
Coenye T, Nelis HJ. In vitro and in vivo model systems to study microbial biofilm formation. J Microbiol Methods. 2010;83(2):89–105.
Chang Y-W, Fragkopoulos AA, Marquez SM, Kim HD, Angelini TE, Fernández-Nieves A. Biofilm formation in geometries with different surface curvature and oxygen availability. New J Phys. 2015;17(3):033017.
Grainger CI, Greenwell LL, Martin GP, Forbes B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur J Pharm Biopharm. 2009;71(2):318–24.
Ong HX, Traini D, Loo C-Y, Sarkissian L, Lauretani G, Scalia S, et al. Is the cellular uptake of respiratory aerosols delivered from different devices equivalent? Eur J Pharm Biopharm. 2015;93:320–7.
Ong HX, Traini D, Cipolla D, Gonda I, Bebawy M, Agus H, et al. Liposomal nanoparticles control the uptake of ciprofloxacin across respiratory epithelia. Pharm Res. 2012;29(12):3335–46.
Abdelrahim ME. Aerodynamic characteristics of nebulized terbutaline sulphate using the Andersen Cascade impactor compared to the next generation impactor. Pharm Dev Technol. 2011;16(2):137–45.
Haghi M, Traini D, Young P. In Vitro Cell Integrated Impactor Deposition Methodology for the Study of Aerodynamically Relevant Size Fractions from Commercial Pressurised Metered Dose Inhalers. Pharm Res. 2014;31(7):1779-87. https://doi.org/10.1007/s11095-013-1282-2.
Ong HX, Traini D, Bebawy M, Young PM. Epithelial profiling of antibiotic controlled release respiratory formulations. Pharm Res. 2011;28(9):2327–38.
Ong HX, Traini D, Salama R, Anderson SD, Daviskas E, Young PM. The effects of mannitol on the transport of ciprofloxacin across respiratory epithelia. Mol Pharm. 2013;10(8):2915–24.
Ong HX, Traini D, Ballerin G, Morgan L, Buddle L, Scalia S, et al. Combined inhaled salbutamol and mannitol therapy for mucus hyper-secretion in pulmonary diseases. AAPS J. 2014;16(2):269–80.
Chua SL, Hultqvist LD, Yuan M, Rybtke M, Nielsen TE, Givskov M, et al. In vitro and in vivo generation and characterization of Pseudomonas Aeruginosa biofilm-dispersed cells via c-di-GMP manipulation. Nat Protoc. 2015;10(8):1165–80.
Administration FaD. Guidance for industry; dissolution testing of immediate release solid oral dosage forms. extended release solid oral dosage forms: development, evaluation and application of in vitro/in vivo correlations 1997.
Moore JW, Flanner HH. Mathematical comparison of dissolution profiles. Pharm Technol. 1996;20(6):64–74.
Administration FaD. Guidance for industry; bioavailability and bioequivalence studies for orally administered drug products—general considerations 2000.
Carvalho TC, Peters JI, Williams RO. Influence of particle size on regional lung deposition–what evidence is there? Int J Pharm. 2011;406:1):1–10.
O'Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997;52(Suppl 2):S31.
Hess D, Fisher D, Williams P, Pooler S, Kacmarek RM. Medication nebulizer performance: effects of diluent volume, nebulizer flow, and nebulizer brand. Chest. 1996;110(2):498–505.
Clark AR. The use of laser diffraction for the evaluation of the aerosol clouds generated by medical nebulizers. Int J Pharm. 1995;115(1):69–78.
Ziegler J, Wachtel H. Comparison of cascade impaction and laser diffraction for particle size distribution measurements. J Aerosol Med. 2005;18(3):311–24.
Ong HX, Traini D, Bebawy M, Young PM. Ciprofloxacin is actively transported across bronchial lung epithelial cells using a Calu-3 air interface cell model. Antimicrob Agents Chemother. 2013;57(6):2535–40.
Cipolla D, Blanchard J, Gonda I. Development of liposomal ciprofloxacin to treat lung infections. Pharmaceutics. 2016;8(1):6.
Willumsen NJ, Davis CW, Boucher RC. Selective response of human airway epithelia to luminal but not serosal solution hypertonicity. Possible role for proximal airway epithelia as an osmolality transducer. J Clin Investig. 1994;94(2):779.
Tseng BS, Zhang W, Harrison JJ, Quach TP, Song JL, Penterman J, et al. The extracellular matrix protects pseudomonas aeruginosa biofilms by limiting the penetration of tobramycin. Environ Microbiol. 2013;15(10):2865–78.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108.
Cotton LA, Graham RJ, Lee RJ. The role of alginate in P. aeruginosa PAO1 biofilm structural resistance to gentamicin and ciprofloxacin. J Exp Microbiol Immunol. 2009;13:58–62.
Suci P, Mittelman M, Yu F, Geesey G. Investigation of ciprofloxacin penetration into Pseudomonas Aeruginosa biofilms. Antimicrob Agents Chemother. 1994;38(9):2125–33.
Nguyen D, Joshi-Datar A, Lepine F, Bauerle E, Olakanmi O, Beer K, et al. Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria. Science. 2011;334(6058):982–6.
Bernier SP, Lebeaux D, DeFrancesco AS, Valomon A, Soubigou G, Coppée J-Y, et al. Starvation, together with the SOS response, mediates high biofilm-specific tolerance to the fluoroquinolone ofloxacin. PLoS Genet. 2013;9(1):e1003144.
Bucior I, Abbott J, Song Y, Matthay MA, Engel JN. Sugar administration is an effective adjunctive therapy in the treatment of Pseudomonas Aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 2013;305(5):L352–63.
Flemming H-C, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loo, CY., Lee, WH., Lauretani, G. et al. Sweetening Inhaled Antibiotic Treatment for Eradication of Chronic Respiratory Biofilm Infection. Pharm Res 35, 50 (2018). https://doi.org/10.1007/s11095-018-2350-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2350-4