ABSTRACT
Purpose
Using human lung cancer cells, we evaluated the involvement of plasminogen activator inhibitor-1 (PAI-1) in the anti-invasive action of cannabidiol, a non-psychoactive cannabinoid.
Methods
Invasion was quantified by a modified Boyden chamber assay. PAI-1 protein in cell culture media and PAI-1 mRNA were determined by immunoblotting and RT-PCR, respectively.
Results
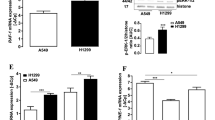
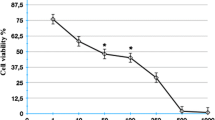
Cannabidiol caused a profound inhibition of A549 cell invasion, accompanied by a decreased expression and secretion of PAI-1. Cannabidiol's effects on PAI-1 secretion and invasion were suppressed by antagonists to CB1 and CB2 receptors as well as to transient receptor potential vanilloid 1. Recombinant human PAI-1 and PAI-1 siRNA led to a concentration-dependent up- and down-regulation of invasiveness, respectively, suggesting a crucial role of PAI-1 in A549 invasiveness. Evidence for a causal link between cannabidiol's effects on PAI-1 and invasion was provided by experiments showing a reversal of its anti-invasive action by addition of recombinant PAI-1 at non-proinvasive concentrations. Key data were confirmed in two other human lung cancer cell lines (H460, H358). In vivo, a significant downregulation of PAI-1 protein by cannabidiol was demonstrated in A549 xenografts.
Conclusion
Our data provide evidence for a hitherto unknown mechanism underlying the anti-invasive action of cannabidiol on human lung cancer cells.








Similar content being viewed by others
Abbreviations
- AM-251:
-
N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- AM-630:
-
(6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl) (4-methoxyphenyl)methanone
- CB1 :
-
cannabinoid receptor 1
- CB2 :
-
cannabinoid receptor 2
- RT-PCR:
-
reverse transcriptase-polymerase chain reaction
- siRNA:
-
small-interfering RNA
- TRPV1:
-
transient receptor potential vanilloid 1
- WST-1:
-
4-[3-(4-Iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1.6-benzene disulfonate
REFERENCES
Munson AE, Harris LS, Friedman MA, Dewey WL, Carchman RA. Antineoplastic activity of cannabinoids. J Natl Cancer Inst. 1975;55:597–602.
Jacobsson SO, Wallin T, Fowler CJ. Inhibition of rat C6 glioma cell proliferation by endogenous and synthetic cannabinoids. Relative involvement of cannabinoid and vanilloid receptors. J Pharmacol Exp Ther. 2001;299:951–9.
Blázquez C, Carracedo A, Barrado L, Real PJ, Fernández-Luna JL, Velasco G, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–5.
Galve-Roperh I, Sánchez C, Cortés ML, Gómez del Pulgar T, Izquierdo M, Guzmán M. Anti-tumoral action of cannabinoids: involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat Med. 2000;6:313–9.
Hinz B, Ramer R, Eichele K, Weinzierl U, Brune K. Up-regulation of cyclooxygenase-2 expression is involved in R(+)-methanandamide-induced apoptotic death of human neuroglioma cells. Mol Pharmacol. 2004;66:1643–51.
Portella G, Laezza C, Laccetti P, De Petrocellis L, Di Marzo V, Bifulco M. Inhibitory effects of cannabinoid CB1 receptor stimulation on tumor growth and metastatic spreading: actions on signals involved in angiogenesis and metastasis. FASEB J. 2003;17:1771–3.
Blázquez C, Casanova ML, Planas A, Gómez Del Pulgar T, Villanueva C, Fernández-Aceñero MJ, et al. Inhibition of tumor angiogenesis by cannabinoids. FASEB J. 2003;17:529–31.
Blázquez C, González-Feria L, Alvarez L, Haro A, Casanova ML, Guzmán M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004;64:5617–23.
Kishimoto S, Muramatsu M, Gokoh M, Oka S, Waku K, Sugiura T. Endogenous cannabinoid receptor ligand induces the migration of human natural killer cells. J Biochem. 2005;137:217–23.
Wang D, Wang H, Ning W, Backlund MG, Dey SK, DuBois RN. Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res. 2008;68:6468–76.
Freimuth N, Ramer R, Hinz B. Antitumorigenic effects of cannabinoids beyond apoptosis. J Pharmacol Exp Ther. 2010;332:336–44.
Grimaldi C, Pisanti S, Laezza C, Malfitano AM, Santoro A, Vitale M, et al. Anandamide inhibits adhesion and migration of breast cancer cells. Exp Cell Res. 2006;312:363–73.
Preet A, Ganju RK, Groopman JE. Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene. 2008;27:339–46.
Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst. 2008;100:59–69.
Ramer R, Merkord J, Rohde H, Hinz B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol. 2010;79:955–66.
Blázquez C, Salazar M, Carracedo A, Lorente M, Egia A, González-Feria L, et al. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008;68:1945–52.
Rog DJ, Nurmikko TJ, Young CA. Oromucosal Δ9- tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther. 2007;29:2068–79.
Ligresti A, Moriello AS, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–87.
McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol Cancer Ther. 2007;6:2921–7.
Vaccani A, Massi P, Colombo A, Rubino T, Parolaro D. Cannabidiol inhibits human glioma cell migration through a cannabinoid receptor-independent mechanism. Br J Pharmacol. 2005;144:1032–6.
McKallip RJ, Jia W, Schlomer J, Warren JW, Nagarkatti PS, Nagarkatti M. Cannabidiol-induced apoptosis in human leukemia cells: a novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol Pharmacol. 2006;70:897–908.
Massi P, Valenti M, Vaccani A, Gasperi V, Perletti G, Marras E, et al. 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J Neurochem. 2008;104:1091–100.
Kogan NM, Blázquez C, Alvarez L, Gallily R, Schlesinger M, Guzmán M, et al. Cannabinoid quinone inhibits angiogenesis by targeting vascular endothelial cells. Mol Pharmacol. 2006;70:51–9.
Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40.
Soff GA, Sanderowitz J, Gately S, Verrusio E, Weiss I, Brem S, et al. Expression of plasminogen activator inhibitor type 1 by human prostate carcinoma cells inhibits primary tumor growth, tumor-associated angiogenesis, and metastasis to lung and liver in an athymic mouse model. J Clin Invest. 1995;96:2593–600.
Roca C, Primo L, Valdembri D, Cividalli A, Declerck P, Carmeliet P, et al. Hyperthermia inhibits angiogenesis by a plasminogen activator inhibitor 1-dependent mechanism. Cancer Res. 2003;63:1500–7.
Chen SC, Henry DO, Hicks DG, Reczek PR, Wong MK. Intravesical administration of plasminogen activator inhibitor type-1 inhibits in vivo bladder tumor invasion and progression. J Urol. 2009;181:336–42.
Bajou K, Noël A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–8.
Bajou K, Maillard C, Jost M, Lijnen RH, Gils A, Declerck P, et al. Host-derived plasminogen activator inhibitor-1 (PAI-1) concentration is critical for in vivo tumoral angiogenesis and growth. Oncogene. 2004;23:6986–90.
Gutierrez LS, Schulman A, Brito-Robinson T, Noria F, Ploplis VA, Castellino FJ. Tumor development is retarded in mice lacking the gene for urokinase type plasminogen activator or its inhibitor, plasminogen activator inhibitor-1. Cancer Res. 2000;60:5839–47.
Binder BR, Mihaly J. The plasminogen activator inhibitor “paradox” in cancer. Immunol Lett. 2008;118:116–24.
Ramer R, Eichele K, Hinz B. Upregulation of tissue inhibitor of matrix metalloproteinases-1 confers the anti-invasive action of cisplatin on human cancer cells. Oncogene. 2007;26:5822–7.
Mukherjee S, Adams M, Whiteaker K, Daza A, Kage K, Cassar S, et al. Species comparison and pharmacological characterization of rat and human CB2 cannabinoid receptors. Eur J Pharmacol. 2004;505:1–9.
Sativex Product Monograph; GW Pharma Ltd. Salisbury, Wiltshire U.K. SP4 0JQ Submission Control No: 091289.
Consroe P, Kennedy K, Schram K. Assay of plasma cannabidiol by capillary gas chromatography/ion trap mass spectroscopy following high-dose repeated daily oral administration in humans. Pharmacol Biochem Behav. 1991;40:517–22.
Thomas BF, Gilliam AF, Burch DF, Roche MJ, Seltzman HH. Comparative receptor binding analyses of cannabinoid agonists and antagonists. J Pharmacol Exp Ther. 1998;285:285–92.
Sacerdote P, Martucci C, Vaccani A, Bariselli F, Panerai AE, Colombo A, et al. The nonpsychoactive component of marijuana cannabidiol modulates chemotaxis and IL-10 and IL-12 production of murine macrophages both in vivo and in vitro. J Neuroimmunol. 2005;159:97–105.
Watanabe K, Kayano Y, Matsunaga T, Yamamoto I, Yoshimura H. Inhibition of anandamide amidase activity in mouse brain microsomes by cannabinoids. Biol Pharm Bull. 1996;19:1109–11.
de Filippis D, Iuvone T, d'Amico A, Esposito G, Steardo L, Herman AG, et al. Effect of cannabidiol on sepsis-induced motility disturbances in mice: involvement of CB receptors and fatty acid amide hydrolase. Neurogastroenterol Motil. 2008;20:919–27.
Costa B, Giagnoni G, Franke C, Trovato AE, Colleoni M. Vanilloid TRPV1 receptor mediates the antihyperalgesic effect of the nonpsychoactive cannabinoid, cannabidiol, in a rat model of acute inflammation. Br J Pharmacol. 2004;143:247–50.
Uddén J, Eriksson P, Hoffstedt J. Glucocorticoid-regulated adipose tissue secretion of PAI-1, but not IL-6, TNFalpha or leptin in vivo. Horm Metab Res. 2002;34:698–702.
Jin H, Lin J, Fu L, Mei YF, Peng G, Tan X, et al. Physiological testosterone stimulates tissue plasminogen activator and tissue factor pathway inhibitor and inhibits plasminogen activator inhibitor type 1 release in endothelial cells. Biochem Cell Biol. 2007;85:246–51.
Yamamoto C, Sugato M, Fujiwara Y, Kaji T. Selective promotion of plasminogen activator inhibitor-1 secretion by activation of proteinase-activated receptor-1 in cultured human brain microvascular pericytes: comparison with endothelial cells. Biol Pharm Bull. 2005;28:208–11.
Yki-Järvinen H, Sutinen J, Silveira A, Korsheninnikova E, Fisher RM, Kannisto K, et al. Regulation of plasma PAI-1 concentrations in HAART-associated lipodystrophy during rosiglitazone therapy. Arterioscler Thromb Vasc Biol. 2003;23:688–94.
Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin αvβ3 binding to vitronectin. Nature. 1996;383:441–3.
Kanse SM, Kost C, Wilhelm OG, Andreasen PA, Preissner KT. The urokinase receptor is a major vitronectin-binding protein on endothelial cells. Exp Cell Res. 1996;224:344–53.
Dong Z, Saliganan AD, Meng H, Nabha SM, Sabbota AL, Sheng S, et al. Prostate cancer cell-derived urokinase-type plasminogen activator contributes to intraosseous tumor growth and bone turnover. Neoplasia. 2008;10:439–49.
ACKNOWLEDGEMENTS
This study was supported by grants from the Deutsche Krebshilfe e.V. (Bonn, Germany).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramer, R., Rohde, A., Merkord, J. et al. Decrease of Plasminogen Activator Inhibitor-1 May Contribute to the Anti-Invasive Action of Cannabidiol on Human Lung Cancer Cells. Pharm Res 27, 2162–2174 (2010). https://doi.org/10.1007/s11095-010-0219-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0219-2