Abstract
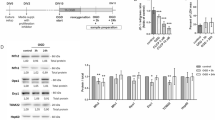
The assembly of complex I (CI) with complexes III (CIII) and IV (CIV) of the mitochondrial respiratory chain (MRC) to configure I–III- or I–III–IV-containing supercomplexes (SCs) regulates mitochondrial energy efficiency and reactive oxygen species (mROS) production. However, whether the occurrence of SCs impacts on CI specific activity remains unknown to our knowledge. To investigate this issue, here we determined CI activity in primary neurons and astrocytes, cultured under identical antioxidants-free medium, from two mouse strains (C57Bl/6 and CBA) and Wistar rat, i.e. three rodent species with or without the ability to assemble CIV into SCs. We found that CI activity was 6- or 1.8-fold higher in astrocytes than in neurons, respectively, from rat or CBA mouse, which can form I–III2–IV SC; however, CI activity was similar in the cells from C57Bl/6 mouse, which does not form I–III2–IV SC. Interestingly, CII–III activity, which was comparable in neurons and astrocytes from mice, was about 50% lower in astrocytes when compared with neurons from rat, a difference that was abolished by antioxidants- or serum-containing media. CIV and citrate synthase activities were similar under all conditions studied. Interestingly, in rat astrocytes, CI abundance in I–III2–IV SC was negligible when compared with its abundance in I–III-containing SCs. Thus, CIV-containing SCs formation may determine CI specific activity in astrocytes, which is important to understand the mechanism for CI deficiency observed in Parkinson’s disease.




Similar content being viewed by others
Abbreviations
- AO:
-
With antioxidants
- BNGE:
-
Blue native gel electrophoresis
- CI:
-
Complex I
- CIII:
-
Complex III
- CIV:
-
Complex IV
- DMEM:
-
Dulbecco’s modified eagle’s medium
- FCS:
-
Fetal calf serum
- MAO:
-
Minus antioxidants
- MRC:
-
Mitochondrial respiratory chain
- mROS:
-
Mitochondrial reactive oxygen species
- PAGE:
-
Polyacrylamide gel electrophoresis
- PBS:
-
Phosphate buffered saline
- SCAF1:
-
Supercomplex assembly factor 1
- SC:
-
Supercomplex
References
Bianchi C, Genova ML, Parenti Castelli G, Lenaz G (2004) The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem 279:36562–36569
Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, Navas P, Cruz R, Carracedo A, Lopez-Otin C, Perez-Martos A, Fernandez-Silva P, Fernandez-Vizarra E, Enriquez JA (2013) Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340:1567–1570
Letts JA, Fiedorczuk K, Sazanov LA (2016) The architecture of respiratory supercomplexes. Nature 537:644–648
Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155:160–171
Enriquez JA (2016) Supramolecular organization of respiratory complexes. Annu Rev Physiol 78:533–561
Cogliati S, Enriquez JA, Scorrano L (2016) Mitochondrial cristae: where beauty meets functionality. Trends Biochem Sci 41:261–273
Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP (2016) Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci USA. doi:10.1073/pnas.1613701113
Bolaños JP, Heales SJR, Land JM, Clark JB (1995) Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary cultures. J Neurochem 64:1965–1972
Stewart VC, Land JM, Clark JB, Heales SJ (1998) Comparison of mitochondrial respiratory chain enzyme activities in rodent astrocytes and neurones and a human astrocytoma cell line. Neurosci Lett 247:201–203
Requejo-Aguilar R, Lopez-Fabuel I, Fernandez E, Martins LM, Almeida A, Bolanos JP (2014) PINK1 deficiency sustains cell proliferation by reprogramming glucose metabolism through HIF1. Nat Commun 5:4514
Jimenez-Blasco D, Santofimia-Castano P, Gonzalez A, Almeida A, Bolanos JP (2015) Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ 22:1877–1889
Ragan CI, Wilson MT, Darley-Usmar VM, Lowe PN (1987) Subfractionation of mitochondria and isolation of the proteins of oxidative phosphorylation. In: Darley-Usmar VM, Rickwood D, Wilson MT (eds) Mitochondria: a practical approach. IRL Press, London, pp 79–112
King TE (1967) Preparation of succinate cytochrome c reductase and the cytochrome b-c1 particle, and reconstitution of succinate cytochrome c reductase. Methods Enzymol 10:216–225
Wharton DC, Tzagoloff A (1967) Cytochrome oxidase from beef heart mitochondria. Methods Enzymol 10:245–250
Shepherd JA, Garland PB (1969) Citrate synthase from rat liver. Methods Enzymol 13:11–19
Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA (2008) Respiratory active mitochondrial supercomplexes. Mol Cell 32:529–539
Diaz F, Barrientos A, Fontanesi F (2009) Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using blue native gel electrophoresis. Curr Protoc Hum Genet 19(19):14
Cogliati S, Calvo E, Loureiro M, Guaras AM, Nieto-Arellano R, Garcia-Poyatos C, Ezkurdia I, Mercader N, Vazquez J, Enriquez JA (2016) Mechanism of super-assembly of respiratory complexes III and IV. Nature. doi:10.1038/nature20157
Tsai MJ, Lee EH (1994) Differences in the disposition and toxicity of 1-methyl-4-phenylpyridinium in cultured rat and mouse astrocytes. Glia 12:329–335
Davey GP, Clark JB (1996) Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem 66:1617–1624
Perry TL, Godin DV, Hansen S (1982) Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett 33:305–310
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269
Schapira AH (2012) Mitochondrial diseases. Lancet 379:1825–1834
Acknowledgements
J.P.B. is funded by MINECO (SAF2013-41177-R, SAF2016-78114-R), CIBER on Frailty and Aging from the Instituto de Salud Carlos III (CB16/10/00282), E.U. SP3-People-MC-ITN programme (608381), EU BATCure Grant (666918) and FEDER (European regional development fund). A.A.P. is funded by the Instituto de Salud Carlos III (RD12/0014/0007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lopez-Fabuel, I., Resch-Beusher, M., Carabias-Carrasco, M. et al. Mitochondrial Complex I Activity is Conditioned by Supercomplex I–III2–IV Assembly in Brain Cells: Relevance for Parkinson’s Disease. Neurochem Res 42, 1676–1682 (2017). https://doi.org/10.1007/s11064-017-2191-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-017-2191-2