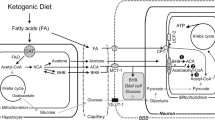
Abstract
β-Hydroxybutyrate (βOHB), a ketone body, is oxidised as a brain fuel. Although its contribution to energy metabolism in the healthy brain is minimal, it is an interesting metabolite which is not only oxidised but also has other direct and collateral effects which make it a molecule of interest for therapeutic purposes. In brain βOHB can be produced in astrocytes from oxidation of fatty acids or catabolism of amino acids and is metabolised in the mitochondria of all brain cell types although uptake across the blood brain barrier is a metabolic control point. βOHB possesses an intrinsic high heat of combustion, making it an efficient mitochondrial fuel, where it can alter the NAD+/NADH and Q/QH2 couples and reduce production of mitochondrial reactive oxygen species. It can directly interact as a signalling molecule influencing opening of K+ channels and regulation of Ca2+ channels. βOHB is an inhibitor of histone deacetylases resulting in upregulation of genes involved in protection against oxidative stress and regulation of metabolism. It interacts with an inflammasome in immune cells to reduce production of inflammatory cytokines and reduce inflammation. Use of βOHB as an efficient neurotherapeutic relies on increasing blood βOHB levels so as to encourage entry of βOHB to the brain. While use of βOHB as a sole therapeutic is currently limited, with employment of a ketogenic diet a more widely used approach, recent development and testing of esterified forms of βOHB have shown great promise, with the approach elevating plasma βOHB while allowing consumption of normal diet. An improved understanding of the mechanisms by which βOHB acts will allow better design of both diet and supplemental interventions.



Similar content being viewed by others
References
Wakeman A, Dakin H (1909) On the decomposition of β-oxybutyric acid and acetoacetic acid by enzymes of the liver. J Biol Chem 6:373–389
Dedkova EN, Blatter LA (2014) Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front Physiol 5:260
Krebs H, Williamson D, Bates MW, Page MA, Hawkins R (1971) The role of ketone bodies in caloric homeostasis. Adv Enzyme Regul 9:387–409
Longo VD, Mattson MP (2014) Fasting: molecular mechanisms and clinical applications. Cell Metab 19:181–192
Gonzalez SV, Nguyen NHT, Rise F, Hassel B (2005) Brain metabolism of exogenous pyruvate. J Neurochem 95:284–293
Dienel GA (2012) Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab 32:1107–1138
Rae C, Fekete AD, Kashem MA, Nasrallah FA, Bröer S (2012) Metabolism, compartmentation, transport and production of acetate in the cortical brain tissue slice. Neurochem Res 37:2541–2553
Künnecke B, Cerdan S, Seelig J (1993) Cerebral metabolism of [1, 2-13C2] glucose and [U-13C4] 3-hydroxybutyrate in rat brain as detected by 13C NMR spectroscopy. NMR Biomed 6:264–277
Edmond J, Robbins R, Bergstrom J, Cole R, De Vellis J (1987) Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 18:551–561
Cahill GF, Veech RL (2003) Ketoacids? Good medicine? Trans Am Clin Climatol Assoc 114:149
Hansen JL, Freier EF (1978) Direct assays of lactate, pyruvate, beta-hydroxybutyrate, and acetoacetate with a centrifugal analyzer. Clin Chem 24:475–479
Owen O, Morgan A, Kemp H, Sullivan J, Herrera M, Cahill G Jr (1967) Brain metabolism during fasting. J Clin Invest 46:1589
Mitchell GA, Kassovska-Bratinova S, Boukaftane Y, Robert MF, Wang SP, Ashmarina L, Lambert M, Lapierre P, Potier E (1995) Medical aspects of ketone body metabolism. Clin Invest Med 18:193–216
Laffel L (1999) Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 15:412–426
Pan JW, Rothman DL, Behar KL, Stein DT, Hetherington HP (2000) Human brain β-hydroxybutyrate and lactate increase in fasting-induced ketosis. J Cereb Blood Flow Metab 20:1502–1507
Samala R, Klein J, Borges K (2011) The ketogenic diet changes metabolite levels in hippocampal extracellular fluid. Neurochem Int 58:5–8
Halestrap AP, Meredith D (2004) The SLC16 gene family—from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflugers Arch 447:619–628
Halestrap AP (2013) Monocarboxylic acid transport. Compr Physiol
Bergersen LH, Magistretti PJ, Pellerin L (2005) Selective postsynaptic co-localisation of MCT2 with AMPA receptor GluR2/3 subunits at excitatory synapses exhibiting AMPA receptor trafficking. Cereb Cortex 15:361–370
Rafiki A, Boulland JL, Halestrap AP, Ottersen OP, Bergersen LH (2003) Highly differential expression of the monocarboxylate transporters MCT2 and MCT4 in the developing rat brain. Neurosci 122:677–688
Broer S, Broer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW (1999) Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J 341:529–535
Carpenter L, Halestrap AP (1994) The kinetics, substrate and inhibitor specificity of the lactate transporter of ehrlich-lettre tumor cells studied with the intracellular pH indicator BCECF. Biochem J 304:751–760
Fox JEM, Meredith D, Halestrap AP (2000) Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol 529:285–293
Martin PM, Gopal E, Ananth S, Zhuang L, Itagaki S, Prasad BM, Smith SB, Prasad PD, Ganapathy V (2006) Identify of SMCT1(SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J Neurochem 98:279–288
Gopal E, Umapathy NS, Martin PM, Ananth S, Gnana-Prakasam JP, Becker H, Wagner CA, Ganapathy V, Prasad PD (2007) Cloning and functional characterization of human SMCT2 (SLC5A12) and expression pattern of the transporter in kidney. Biochim Et Biophys Acta Biomembr 1768:2690–2697
Tildon JT, McKenna MC, Stevenson JH Jr (1994) Transport of 3-hydroxybutyrate by cultured rat brain astrocytes. Neurochem Res 19:1237–1242
Leino RL, Gerhart DZ, Duelli R, Enerson BE, Drewes LR (2001) Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem Int 38:519–527
Regen DM, Callis JT, Sugden MC (1983) Studies of cerebral β-hydroxybutyrate transport by carotid injection; effects of age, diet and injectant composition. Brain Res 271:289–299
Puchowicz MA, Xu K, Sun X, Ivy A, Emancipator D, LaManna JC (2007) Diet-induced ketosis increases capillary density without altered blood flow in rat brain. Am J Physiol Endocrinol Metab 292:E1607
Mikkelsen KH, Seifert T, Secher NH, Grøndal T, van Hall G (2014) Systemic, cerebral and skeletal muscle ketone body and energy metabolism during acute hyper-D-β-hydroxybutyratemia in post-absorptive healthy males. J Clin Endocrinol Metab 100:636–643
Courchesne-Loyer A, Croteau E, Castellano C-A, St-Pierre V, Hennebelle M, Cunnane SC (2016) Inverse relationship between brain glucose and ketone metabolism in adults during short-term moderate dietary ketosis: a dual tracer quantitative positron emission tomography study. J Cereb Blood Flow Metab. doi:10.1177/0271678X16669366
Hawkins RA, Mans AM, Davis DW (1986) Regional ketone body utilization by rat brain in starvation and diabetes. Am J Physiol Endocrinol Metab 250:E169
Ito K, Uchida Y, Ohtsuki S, Aizawa S, Kawakami H, Katsukura Y, Kamiie J, Terasaki T (2011) Quantitative membrane protein expression at the blood–brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci 100:3939–3950
Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR (1997) Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol Endocrinol Metab 273:E207–E213
Vannucci SJ, Simpson IA (2003) Developmental switch in brain nutrient transporter expression in the rat. Am J Physiol Endocrinol Metab 285:E1127–E1134
Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T (2011) Quantitative targeted absolute proteomics of human blood–brain barrier transporters and receptors. J Neurochem 117:333–345
Halestrap AP (1978) Pyruvate and ketone-body transport across the mitochondrial membrane. Exchange properties, pH-dependence and mechanism of the carrier. Biochem J 172:377–387
Paradies G, Papa S (1997) On the kinetics and substrate specificity of the pyruvate translocator in rat liver mitochondria. Biochim Et Biophys Acta Bioenerg 462:333–346
Booth RF, Clark JB (1981) Energy metabolism in rat brain: inhibition of pyruvate decarboxylation by 3-hydroxybutyrate in neonatal mitochondria. J Neurochem 37:179–185
Veech RL (2004) The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandin Leukotriene Essent Fatty Acid 70:309–319
Sato K, Kashiwaya Y, Keon C, Tsuchiya N, King M, Radda G, Chance B, Clarke K, Veech R (1995) Insulin ketone bodies, and mitochondrial energy transduction. FASEB J 9:651–658
Klee CB, Sokoloff L (1967) Changes in d(−)-β-hydroxybutyric dehydrogenase activity during brain maturation in the rat. J Biol Chem 242:3880–3883
Latruffe N, Gaudemer Y (1974) Propriétés et mécanisme cinétique de la D(−)β-hydroxybutyrique déshydrogénase de particules sous-mitochondriales de foie de rat; Effets comparés de différents agents thiols. Biochimie 56:435–444
Rardin MJ, Newman JC, Held JM, Cusack MP, Sorensen DJ, Li B, Schilling B, Mooney SD, Kahn CR, Verdin E (2013) Label-free quantitative proteomics of the lysine acetylome in mitochondria identifies substrates of SIRT3 in metabolic pathways. Proc Natl Acad Sci USA 110:6601–6606
Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM Coon JJ (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49:186–199
Guo K, Lukacik P, Papagrigoriou E, Meier M, Lee WH, Adamski J, Oppermann U (2006) Characterization of human DHRS6, an orphan short chain dehydrogenase/reductase enzyme: a novel, cytosolic type 2 R-beta-hydroxybutyrate dehydrogenase. J Biol Chem 281:10291–10297
Chang HT, Olson LK, Schwartz KA (2013) Ketolytic and glycolytic enzymatic expression profiles in malignant gliomas: implication for ketogenic diet therapy. Nutr Metab 10:1
Bixel MG, Hamprecht B (1995) Generation of ketone bodies from leucine by cultured astroglial cells. J Neurochem 65:2450–2461
Puisac B, Ramos M, Arnedo M, Menao S, Gil-Rodríguez MC, Teresa-Rodrigo ME, Pié A, de Karam JC, Wesselink J-J, Giménez I, Ramos FJ, Casals N, Gómez-Puertas P, Hegardt FG, Pié J (2012) Characterization of splice variants of the genes encoding human mitochondrial HMG-CoA lyase and HMG-CoA synthase, the main enzymes of the ketogenesis pathway. Mol Biol Rep 39:4777–4785
Fukao T, Song X-Q, Mitchell GA, Yamaguchi S, Sukegawa K, Or T, Kondo N (1997) Enzymes of ketone body utilization in human tissues: protein and messenger RNA levels of succinyl-coenzyme A (CoA):3-ketoacid CoA transferase and mitochondrial and cytosolic acetoacetyl-CoA thiolases. Pediatr Res 42:498–502
Ohnuki M, Takahashi N, Yamasaki M, Fukui T (2005) Different localization in rat brain of the novel cytosolic ketone body-utilizing enzyme, acetoacetyl-CoA synthetase, as compared to succinyl-CoA:3-oxoacid CoA-transferase. Biochim Et Biophys Acta 1729:147–153
Endemann G, Goetz PG, Edmond J, Brunengraber H (1982) Lipogenesis from ketone bodies in the isolated perfused rat liver. Evidence for the cytosolic activation of acetoacetate. J Biol Chem 257:3434–3440
Hasegawa S, Kume H, Iinuma S, Yamasaki M, Takahashi N, Fukui T (2012) Acetoacetyl-CoA synthetase is essential for normal neuronal development. Biochem Biophys Res Commun 427:398–403
Tildon JT, Cornblath M (1972) Succinyl-CoA: 3-ketoacid CoA-transferase deficiency. A cause for ketoacidosis in infancy. J Clin Invest 51:493–498
Fukao T, Sass JO, Kursula P, Thimm E, Wendel U, Ficicioglu C, Monastiri K, Guffon N, Barić I, Zabot MT, Kondo N (2011) Clinical and molecular characterization of five patients with succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency. Biochim Et Biophys Acta 1812:619–624
Fukao T, Mitchell GA, Song X-Q, Nakamura H, Kassovska-Bratinova S, Orii KE, Wraith JE, Besley G, Wanders RJA, Niezen-Koning KE, Berry GT, Palmieri M, Kondo N (2000) Succinyl-CoA:3-ketoacid CoA transferase (SCOT): cloning of the human SCOT gene, tertiary structural modeling of the human SCOT monomer, and characterization of three pathogenic mutations. Genomics 68:144–151
Berry G, Fukao T, Mitchell G, Mazur A, Ciafre M, Gibson J, Kondo N, Palmieri M (2001) Neonatal hypoglycaemia in severe succinyl-CoA: 3-oxoacid CoA-transferase deficiency. J Inherit Metab Dis 24:587–595
Gibson K, Breuer J, Nyhan W (1988) 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: review of 18 reported patients. Eur J Pediatr 148:180–186
Van der Knaap M, Bakker H, Valk J (1998) MR imaging and proton spectroscopy in 3-hydroxy-3-methylglutaryl coenzyme A lyase deficiency. Am J Neuroradiol 19:378–382
Gordon K, Riding M, Camfield P, Bawden H, Ludman M, Bagnell P (1994) CT and MR of 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency. Am J Neuroradiol 15:1474–1476
Fukao T, Scriver CR, Kondo N; Group TCW (2001) The clinical phenotype and outcome of mitochondrial acetoacetyl-CoA thiolase deficiency (β-ketothiolase or T2 deficiency) in 26 enzymatically proved and mutation-defined patients. Mol Genet Metab 72:109–114
Bennett M, Hosking G, Smith M, Gray R, Middleton B (1984) Biochemical investigations on a patient with a defect in cytosolic acetoacetyl-CoA thiolase, associated with mental retardation. J Inherit Metab Dis 7:125–128
Hawkins R, Williamson D, Krebs H (1971) Ketone-body utilization by adult and suckling rat brain in vivo. Biochem J 122:13–18
Sokoloff L (1973) Metabolism of ketone bodies by the brain. Annu Rev Med 24:271–280
Cahill GFJ (2006) Fuel metabolism in starvation. Annu Rev Nutr 26:1–22
DeVivo DC, Leckie MP, Agrawal HC (1973) The differential incorporation of β-hydroxybutyrate and glucose into brain glutamate in the newborn rat. Brain Res 55:485–490
Nehlig A (2004) Brain uptake and metabolism of ketone bodies in animal models. Prostaglandin Leukotriene Essent Fatty Acid 70:265–275
Hotta SS (1962) Glucose metabolism in brain tissue: the hexose monophosphate shunt and its role in glutathione reduction. J Neurochem 9:43–51
Bilger A, Nehlig A (1992) Quantitative histochemical changes in enzymes involved in energy metabolism in the rat brain during postnatal development 2. Glucose-6-phosphate dehydrogenase and beta-hydroxybutyrate dehydrogenase. Int J Dev Neurosci 10:143–152
Nehlig A, de Vasconcelos AP (1993) Glucose and ketone body utilization by the brain of neonatal rats. Prog Neurobiol 40:163–220
Rho JM, Stafstrom CE (2012) The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol 3:59
Maalouf M, Rho JM, Mattson MP (2009) The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies. Brain Res Rev 59:293–315
McKenna MC, Tildon JT, Stevenson JH, Boatright R, Huang S (1993) Regulation of energy metabolism in synaptic terminals and cultured rat brain astrocytes: differences revealed using aminooxyacetate. Dev Neurosci 15:320–329
Chechik T, Roeder L, Tildon J, Poduslo S (1987) Ketone body enzyme activities in purified neurons, astrocytes and oligodendroglia. Neurochem Int 10:95–99
Berl S, Frigyesi TL (1969) Turnover of glutamate glutamine aspartate and GABA labeled with 1-14C acetate in caudate nucleus thalamus and motor cortex (cat). Brain Res 12:444–455
Berl S, Nicklas WJ, Clarke DD (1970) Compartmentation of citric acid cycle metabolism in brain-labeilling of glutamate, glutamine, aspartate and GABA by several radioactive tracer metabolites. J Neurochem 17:1009–1015
Cremer JE (1971) Incorporation of label from d-β-hydroxy-[14C] butyrate and [3-14C] acetoacetate into amino acids in rat brain in vivo. Biochem J 122:135–138
Pan JW, de Graaf RA, Petersen KF, Shulman GI, Hetherington HP, Rothman DL (2002) [2,4-13C2]-hydroxybutyrate metabolism in human brain. J Cereb Blood Flow Metab 22:890–898
Leong SF, Lai JCK, Lim L, Clark JB (1981) Energy-metabolising enzymes in brain regions of adult and aging rats. J Neurochem 37:1548–1556
Williamson D, Bates MW, Page MA, Krebs H (1971) Activities of enzymes involved in acetoacetate utilization in adult mammalian tissues. Biochem J 121:41–47
Page MA, Williamson D (1971) Enzymes of ketone-body utilisation in human brain. Lancet 298:66–68
Nugent S, Tremblay S, Chen KW, Ayutyanont N, Roontiva A, Castellano C-A, Fortier M, Roy M, Courchesne-Loyer A, Bocti C (2014) Brain glucose and acetoacetate metabolism: a comparison of young and older adults. Neurobiol Aging 35:1386–1395
Blomqvist G, Thorell J, Ingvar M, Grill V, Widen L, Stone-Elander S (1995) Use of R-beta-[1-11C] hydroxybutyrate in PET studies of regional cerebral uptake of ketone bodies in humans. Am J Physiol Endocrinol Metab 269:E948–E959
Hawkins RA, Biebuyck JF (1979) Ketone bodies are selectively used by individual brain regions. Science 205:325–327
Gesink DS, Wilson JE A (1985) Histochemical study of the distribution of β-hydroxybutyrate dehydrogenase in developing rat cerebellum. J Neurochem 44:1308–1311
Arakawa T, Goto T, Okada Y (1991) Effect of ketone body (D-3-hydroxybutyrate) on neural activity and energy metabolism in hippocampal slices of the adult guinea pig. Neurosci Lett 130:53–56
Wada H, Okada Y, Nabetani M, Nakamura H (1997) The effects of lactate and beta-hydroxybutyrate on the energy metabolism and neural activity of hippocampal slices from adult and immature rat. Dev Brain Res 101:1–7
Weiss JN, Lamp ST (1989) Cardiac ATP-sensitive K+ channels. Evidence for preferential regulation by glycolysis. J Gen Physiol 94:911–935
Dhar-Chowdhury P, Harrell MD, Han SY, Jankowska D, Parachuru L, Morrissey A, Srivastava S, Liu W, Malester B, Yoshida H (2005) The glycolytic enzymes, glyceraldehyde-3-phosphate dehydrogenase, triose-phosphate isomerase, and pyruvate kinase are components of the KATP channel macromolecular complex and regulate its function. J Biol Chem 280:38464–38470
Silver IA, Deas J, Erecinska M (1997) Ion homeostasis in brain cells: differences in intracellular ion reponses to energy limitation between cultured neurons and glial cells. Neuroscience 78:598–601
Chowdhury GM, Jiang L, Rothman DL, Behar KL (2014) The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo. J Cereb Blood Flow Metab 34:1233–1242
Wieland O, Funcke Hv, Löffler G (1971) Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett 15:295–298
Newsholme EA, Randle PJ, Manchester KL (1962) Inhibition of the phosphofructokinase reaction in perfused rat heart by respiration of ketone bodies, fatty acids and pyruvate. Nature 193:270–271
Tildon JT, Roeder LM, Stevenson JH (1985) Substrate oxidation by isolated rat brain mitochondria and synaptosomes. J Neurosci Res 14:207–215
Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP (2016) 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. doi:10.1111/jnc.13868
Erecinska M, Nelson D, Daikhin Y, Yudkoff M (1996) Regulation of GABA level in rat brain synaptosomes: fluxes through enzymes of the GABA shunt and effects of glutamate, calcium and ketone bodies. J Neurochem 67:2325–2334
Yudkoff M, Daikhin Y, Nissim I, Grunstein R, Nissim I (1997) Effects of ketone bodies on astrocyte amino acid metabolism. J Neurochem 69:682–692
McKenna MC, Tildon JT, Stevenson J, Huang X, Kingwell KG (1995) Regulation of mitochondrial and cytosolic malic enzymes from cultured rat brain astrocytes. Neurochem Res 20:1491–1501
Lajtha A, Gibson G, Dienel G (2007) Handbook of neurochemistry and molecular neurobiology. Neuroactive proteins and peptides. Springer, Springer-Verlag
Amaral AI, Teixeira AP, Sonnewald U, Alves PM (2011) Estimation of intracellular fluxes in cerebellar neurons after hypoglycemia: importance of the pyruvate recycling pathway and glutamine oxidation. J Neurosci Res 89:700–710
Lund TM, Risa O, Sonnewald U, Schousboe A, Waagepetersen HS (2009) Availability of neurotransmitter glutamate is diminished when beta-hydroxybutyrate replaces glucose in cultured. J Neurochem 110:80–91
Cruz NF, Lasater A, Zielke HR, Dienel GA (2005) Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem 92:934–947
Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE IV (1987) MPTP, MPP+ and mitochondrial function. Life Sci 40:721–729
Tieu K, Perier C, Caspersen C, Teismann P, Wu D-C, Yan S-D, Naini A, Vila M, Jackson-Lewis V, Ramasamy R (2003) D-β-Hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest 112:892–901
Edwards C, Canfield J, Copes N, Rehan M, Lipps D, Bradshaw PC (2014) D-beta-hydroxybutyrate extends lifespan in C. elegans. Aging 6:621
Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM (2006) A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci 7:1
Kashiwaya Y, Takeshima T, Mori N, Nakashima K, Clarke K, Veech RL (2000) d-β-Hydroxybutyrate protects neurons in models of Alzheimer’s and Parkinson’s disease. Proc Natl Acad Sci USA 97:5440–5444
Julio-Amilpas A, Montiel T, Soto-Tinoco E, Gerónimo-Olvera C, Massieu L (2015) Protection of hypoglycemia-induced neuronal death by β-hydroxybutyrate involves the preservation of energy levels and decreased production of reactive oxygen species. J Cereb Blood Flow Metab 35:851–860
McKenna M, Tildon J, Stevenson J, Hopkins I (1994) Energy metabolism in cortical synaptic terminals from weanling and mature rat brain: evidence for multiple compartments of tricarboxylic acid cycle activity. Dev Neurosci 16:291–300
Lund TM, Ploug KB, Iversen A, Jensen AA, Jansen-Olesen I (2015) The metabolic impact of β-hydroxybutyrate on neurotransmission: reduced glycolysis mediates changes in calcium responses and KATP channel receptor sensitivity. J Neurochem 132:520–531
Ma W, Berg J, Yellen G (2007) Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci 27:3618–3625
Giménez-Cassina A, Martínez-François Juan R, Fisher Jill K, Szlyk B, Polak K, Wiwczar J, Tanner Geoffrey R, Lutas A, Yellen G (2012) Danial Nika N BAD-dependent regulation of fuel metabolism and KATP channel activity confers resistance to epileptic seizures. Neuron 74:719–730
Xiao X-Q, Zhao Y, Chen G-Q (2007) The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 28:3608–3616
Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108:8030–8035
Won Y-J, Lu VB, Puhl HL, Ikeda SR (2013) β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3. J Neurosci 33:19314–19325
Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD (2013) Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339:211–214
Mejía-Toiber J, Montiel T, Massieu L (2006) D-β-hydroxybutyrate prevents glutamate-mediated lipoperoxidation and neuronal damage elicited during glycolysis inhibition in vivo. Neurochem Res 31:1399–1408
Yamada T, Zhang S-J, Westerblad H, Katz A (2010) β-Hydroxybutyrate inhibits insulin-mediated glucose transport in mouse oxidative muscle. Am J Physiol Endocrinol Metab 299:E364–E373
Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD (2015) Ketone body β-hydroxybutyrate blocks the NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21:263–269
Tschopp J, Schroder K (2010) NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol 10:210–215
Shao B-Z, Xu Z-Q, Han B-Z, Su D-F, Liu C (2015) NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 6:262
Lee G-S, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ (2012) The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature 492:123–127
Frahm J, Merboldt KD, Hanicke W (1987) Localized proton spectroscopy using stimulated echoes. J Magn Reson 72:502–508
Kreis R, Ross B (1992) Cerebral metabolic disturbances in patients with subacute and chronic diabetes mellitus: detection with proton MR spectroscopy. Radiology 184:123–130
Plecko B, Stoeckler-Ipsiroglu S, Schober E, Harrer G, Mlynarik V, Gruber S, Moser E, Moeslinger D, Silgoner H, Ipsiroglu O (2002) Oral &bgr;-hydroxybutyrate supplementation in two patients with hyperinsulinemic hypoglycemia: monitoring of &bgr;-hydroxybutyrate levels in blood and cerebrospinal fluid, and in the brain by in vivo magnetic resonance spectroscopy. Pediatric Res 52:301–306
Shen J, Novotny EJ, Rothman DL (1998) In vivo lactate and β-hydroxybutyrate editing using a pure-phase refocusing pulse train. Magn Reson Med 40:783–788
Ordidge R, Connelly A, Lohman J (1969) Image-selected in vivo spectroscopy (ISIS). A new technique for spatially selective NMR spectroscopy. J Magn Reson 66:283–294
Wootton-Gorges SL, Buonocore MH, Kuppermann N, Marcin J, DiCarlo J, Neely EK, Barnes PD, Glaser N (2005) Detection of cerebral β-hydroxy butyrate, acetoacetate, and lactate on proton MR spectroscopy in children with diabetic ketoacidosis. Am J Neuroradiol 26:1286–1291
Cecil KM, Mulkey SB, Ou X, Glasier CM (2015) Brain ketones detected by proton magnetic resonance spectroscopy in an infant with Ohtahara syndrome treated with ketogenic diet. Pediatr Radiol 45:133–137
Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R (1998) Simultaneous in vivo spectral editing and water suppression. NMR Biomed 11:266–272
Edden RAE, Harris AD, Murphy K, Evans CJ, Saxena N, Hall JE, Bailey DM, Wise RG (2010) Edited MRS is sensitive to changes in lactate concentration during inspiratory hypoxia. J Magn Reson Imag 32:320–325
Courchesne-Loyer A, Fortier M, Tremblay-Mercier J, Chouinard-Watkins R, Roy M, Nugent S, Castellano C-A, Cunnane SC (2013) Stimulation of mild, sustained ketonemia by medium-chain triacylglycerols in healthy humans: estimated potential contribution to brain energy metabolism. Nutrition 29:635–640
Reger MA, Henderson ST, Hale C, Cholerton B, Baker LD, Watson G, Hyde K, Chapman D, Craft S (2004) Effects of β-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging 25:311–314
Clarke K, Tchabanenko K, Pawlosky R, Carter E, King MT, Musa-Veloso K, Ho M, Roberts A, Robertson J, VanItallie TB (2012) Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol 63:401–408
Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL (2015) A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement 11:99–103
Hertz L, Chen Y, Waagepetersen HS (2015) Effects of ketone bodies in Alzheimer’s disease in relation to neural hypometabolism, β-amyloid toxicity, and astrocyte function. J Neurochem 134:7–20
Orhan N, Ugur Yilmaz C, Ekizoglu O, Ahishali B, Kucuk M, Arican N, Elmas I, Gürses C, Kaya M (2016) Effects of beta-hydroxybutyrate on brain vascular permeability in rats with traumatic brain injury. Brain Res 1631:113–126
Jarrett SG, Milder JB, Liang LP, Patel M (2008) The ketogenic diet increases mitochondrial glutathione levels. J Neurochem 106:1044–1051
Prins M, Fujima L, Hovda D (2005) Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res 82:413–420
Bröer S, Schneider HP, Bröer A, Rahman B, Hamprecht B, Deitmer JW (1998) Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J 333:167–174
Acknowledgements
The authors would like to thank Ben Rowlands of Neuroscience Research Australia and Don Thomas of the UNSW Mark Wainwright Analytical Centre for their assistance in the preparation of this manuscript. The authors would like to acknowledge the lifetime commitment of Professor Mary McKenna to the pursuit of scientific excellence and education in neurochemistry and to dedicate this article to her contributions to this field. We have greatly enjoyed and also benefitted from our interactions over the years.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Achanta, L.B., Rae, C.D. β-Hydroxybutyrate in the Brain: One Molecule, Multiple Mechanisms. Neurochem Res 42, 35–49 (2017). https://doi.org/10.1007/s11064-016-2099-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2099-2