Abstract
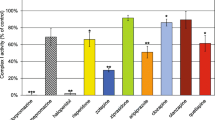
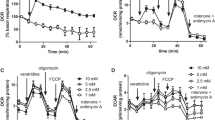
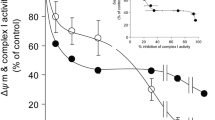
The aim of this work was to characterize the effects of partial inhibition of respiratory complex I by rotenone on H2O2 production by isolated rat brain mitochondria in different respiratory states. Flow cytometric analysis of membrane potential in isolated mitochondria indicated that rotenone leads to uniform respiratory inhibition when added to a suspension of mitochondria. When mitochondria were incubated in the presence of a low concentration of rotenone (10 nm) and NADH-linked substrates, oxygen consumption was reduced from 45.9 ± 1.0 to 26.4 ± 2.6 nmol O2 mg−1 min−1 and from 7.8 ± 0.3 to 6.3 ± 0.3 nmol O2 mg−1 min−1 in respiratory states 3 (ADP-stimulated respiration) and 4 (resting respiration), respectively. Under these conditions, mitochondrial H2O2 production was stimulated from 12.2 ± 1.1 to 21.0 ± 1.2 pmol H2O2 mg−1 min−1 and 56.5 ± 4.7 to 95.0 ± 11.1 pmol H2O2 mg−1 min−1 in respiratory states 3 and 4, respectively. Similar results were observed when comparing mitochondrial preparations enriched with synaptic or nonsynaptic mitochondria or when 1-methyl-4-phenylpyridinium ion (MPP+) was used as a respiratory complex I inhibitor. Rotenone-stimulated H2O2 production in respiratory states 3 and 4 was associated with a high reduction state of endogenous nicotinamide nucleotides. In succinate-supported mitochondrial respiration, where most of the mitochondrial H2O2 production relies on electron backflow from complex II to complex I, low rotenone concentrations inhibited H2O2 production. Rotenone had no effect on mitochondrial elimination of micromolar concentrations of H2O2. The present results support the conclusion that partial complex I inhibition may result in mitochondrial energy crisis and oxidative stress, the former being predominant under oxidative phosphorylation and the latter under resting respiration conditions.







Similar content being viewed by others
References
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD (1989) Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1:1269
Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145
Parker WD Jr, Boyson SJ, Parks JK (1989) Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol 26:719–723
Schapira AH (1994) Evidence for mitochondrial dysfunction in Parkinson’s disease—a critical appraisal. Mov Disord 9:125–138
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38:515–517
Schon EA, DiMauro S, Hirano M (2012) Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet 13:878–890
Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE (1987) MPTP, MPP+ and mitochondrial function. Life Sci 40:721–729
Langston JW, Ballard P, Tetrud JW, Irwin I (1983) Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219:979–980
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3:1301–1306
Greenamyre JT, Cannon JR, Drolet R, Mastroberardino PG (2010) Lessons from the rotenone model of Parkinson’s disease. Trends Pharmacol Sci 31:141–142
Tapias V, Cannon JR, Greenamyre JT (2010) Melatonin treatment potentiates neurodegeneration in a rat rotenone Parkinson’s disease model. J Neurosci Res 88:420–427
Sanders LH, Timothy Greenamyre J (2013) Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic Biol Med 62:111–120
Barrientos A, Moraes CT (1999) Titrating the effects of mitochondrial complex I impairment in the cell physiology. J Biol Chem 274:16188–16197
Chinopoulos C, Adam-Vizi V (2001) Mitochondria deficient in complex I activity are depolarized by hydrogen peroxide in nerve terminals: relevance to Parkinson’s disease. J Neurochem 76:302–306
Testa CM, Sherer TB, Greenamyre JT (2005) Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res 134:109–118
Yadava N, Nicholls DG (2007) Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci 27:7310–7317
Pöltl D, Schildknecht S, Karreman C, Leist M (2012) Uncoupling of ATP-depletion and cell death in human dopaminergic neurons. Neurotoxicology 33:769–779
Surmeier DJ, Schumacker PT (2013) Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J Biol Chem 288:10736–10741
Votyakova TV, Reynolds IJ (2001) DeltaPsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J Neurochem 79:266–277
Liu Y, Fiskum G, Schubert D (2002) Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787
Sousa SC, Maciel EN, Vercesi AE, Castilho RF (2003) Ca2+-induced oxidative stress in brain mitochondria treated with the respiratory chain inhibitor rotenone. FEBS Lett 543:179–183
Tahara EB, Navarete FD, Kowaltowski AJ (2009) Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic Biol Med 46:1283–1297
Starkov AA, Fiskum G (2003) Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem 86:1101–1107
Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279:4127–4135
Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT (2002) An in vitro model of Parkinson’s disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci 22:7006–7015
Sipos I, Tretter L, Adam-Vizi V (2003) Quantitative relationship between inhibition of respiratory complexes and formation of reactive oxygen species in isolated nerve terminals. J Neurochem 84:112–118
Mirandola SR, Melo DR, Saito A, Castilho RF (2010) 3-nitropropionic acid-induced mitochondrial permeability transition: comparative study of mitochondria from different tissues and brain regions. J Neurosci Res 88:630–639
Rosenthal RE, Hamud F, Fiskum G, Varghese PJ, Sharpe S (1987) Cerebral ischemia and reperfusion: prevention of brain mitochondrial injury by lidoflazine. J Cereb Blood Flow Metab 7:752–758
Sims NR, Anderson MF (2008) Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat Protoc 3:1228–1239
Sims NR (1990) Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem 55:698–707
Kaplan RS, Pedersen PL (1983) Characterization of phosphate efflux pathways in rat liver mitochondria. Biochem J 212:279–288
Mattiasson G (2004) Flow cytometric analysis of isolated liver mitochondria to detect changes relevant to cell death. Cytom A 60:145–154
Robinson J, Cooper JM (1970) Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem 33:390–399
Tretter L, Biagioni Angeli E, Ardestani MR, Goracci G, Adam-Vizi V (2011) Reversible inhibition of hydrogen peroxide elimination by calcium in brain mitochondria. J Neurosci Res 89:1965–1972
Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HC, Vercesi AE, Castilho RF (2013) A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med 63:446–456
Long J, Ma J, Luo C, Mo X, Sun L, Zang W, Liu J (2009) Comparison of two methods for assaying complex I activity in mitochondria isolated from rat liver, brain and heart. Life Sci 85:276–280
Navarro A, Bández MJ, Gómez C, Repetto MG, Boveris A (2010) Effects of rotenone and pyridaben on complex I electron transfer and on mitochondrial nitric oxide synthase functional activity. J Bioenerg Biomembr 42:405–412
Agarwal B, Dash RK, Stowe DF, Bosnjak ZJ, Camara AK (2014) Isoflurane modulates cardiac mitochondrial bioenergetics by selectively attenuating respiratory complexes. Biochim Biophys Acta 1837:354–365
Aiuchi T, Shirane Y, Kinemuchi H, Arai Y, Nakaya K, Nakamura Y (1988) Enhancement by tetraphenylboron of inhibition of mitochondrial respiration induced by 1-methyl-4-phenylpyridinium ion (MPP+). Neurochem Int 12:525–531
Ramsay RR, Mehlhorn RJ, Singer TP (1989) Enhancement by tetraphenylboron of the interaction of the 1-methyl-4-phenylpyridinium ion (MPP+) with mitochondria. Biochem Biophys Res Commun 159:983–990
Chinta SJ, Rane A, Yadava N, Andersen JK, Nicholls DG, Polster BM (2009) Reactive oxygen species regulation by AIF- and complex I-depleted brain mitochondria. Free Radic Biol Med 46:939–947
Gyulkhandanyan AV, Pennefather PS (2004) Shift in the localization of sites of hydrogen peroxide production in brain mitochondria by mitochondrial stress. J Neurochem 90:405–421
Figueira TR, Barros MH, Camargo AA, Castilho RF, Ferreira JC, Kowaltowski AJ, Sluse FE, Souza-Pinto NC, Vercesi AE (2013) Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxid Redox Signal 18:2029–2074
Korshunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18
Petrosillo G, Ruggiero FM, Paradies G (2003) Role of reactive oxygen species and cardiolipin in the release of cytochrome c from mitochondria. FASEB J 17:2202–2208
Zoccarato F, Cavallini L, Alexandre A (2004) Respiration-dependent removal of exogenous H2O2 in brain mitochondria: inhibition by Ca2+. J Biol Chem 279:4166–4174
Lopert P, Patel M (2014) Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem 289:15611–15620
Rossignol R, Malgat M, Mazat JP, Letellier T (1999) Threshold effect and tissue specificity. Implication for mitochondrial cytopathies. J Biol Chem 274:33426–33432
Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ (2007) ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447:1081–1086
Fahn S, Cohen G (1992) The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol 32:804–812
Jenner P, Dexter DT, Sian J, Schapira AH, Marsden CD (1992) Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann Neurol 32(Suppl):S82–S87
Camara AK, Lesnefsky EJ, Stowe DF (2010) Potential therapeutic benefits of strategies directed to mitochondria. Antioxid Redox Signal 13:279–347
Perfeito R, Cunha-Oliveira T, Rego AC (2013) Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse. Free Radic Biol Med 62:186–201
Brand MD (2010) The sites and topology of mitochondrial superoxide production. Exp Gerontol 2010(45):466–472
Ramsay RR, Krueger MJ, Youngster SK, Gluck MR, Casida JE, Singer TP (1991) Interaction of 1-methyl-4-phenylpyridinium ion (MPP+) and its analogs with the rotenone/piericidin binding site of NADH dehydrogenase. J Neurochem 56:1184–1190
Gerlach M, Riederer P, Przuntek H, Youdim MB (1991) MPTP mechanisms of neurotoxicity and their implications for Parkinson’s disease. Eur J Pharmacol 208:273–286
Tipton KF, Singer TP (1993) Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J Neurochem 61:1191–1206
Bajpai P, Sangar MC, Singh S, Tang W, Bansal S, Chowdhury G, Cheng Q, Fang JK, Martin MV, Guengerich FP, Avadhani NG (2013) Metabolism of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by mitochondrion-targeted cytochrome P450 2D6: implications in Parkinson disease. J Biol Chem 288:4436–4451
Hattori N, Tanaka M, Ozawa T, Mizuno Y (1991) Immunohistochemical studies on complexes I, II, III, and IV of mitochondria in Parkinson’s disease. Ann Neurol 30:563–571
McNaught KS, Jenner P (1999) Altered glial function causes neuronal death and increases neuronal susceptibility to 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced toxicity in astrocytic/ventral mesencephalic co-cultures. J Neurochem 73:2469–2476
Schapira AH (1999) Mitochondrial involvement in Parkinson’s disease, Huntington’s disease, hereditary spastic paraplegia and Friedreich’s ataxia. Biochim Biophys Acta 1410:159–170
Brown MR, Sullivan PG, Geddes JW (2006) Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem 281:11658–11668
Naga KK, Sullivan PG, Geddes JW (2007) High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci 27:7469–7475
Lores-Arnaiz S, Bustamante J (2011) Age-related alterations in mitochondrial physiological parameters and nitric oxide production in synaptic and non-synaptic brain cortex mitochondria. Neuroscience 188:117–124
Davey GP, Clark JB (1996) Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem 66:1617–1624
Davey GP, Peuchen S, Clark JB (1998) Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem 273:12753–12757
Cino M, Del Maestro RF (1989) Generation of hydrogen peroxide by brain mitochondria: the effect of reoxygenation following postdecapitative ischemia. Arch Biochem Biophys 269:623–638
Pryde KR, Hirst J (2011) Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer. J Biol Chem 286:18056–18065
Treberg JR, Quinlan CL, Brand MD (2011) Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). J Biol Chem 286:27103–27110
Orr AL, Ashok D, Sarantos MR, Shi T, Hughes RE, Brand MD (2013) Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free Radic Biol Med 65C:1047–1059
Vesce S, Kirk L, Nicholls DG (2004) Relationships between superoxide levels and delayed calcium deregulation in cultured cerebellar granule cells exposed continuously to glutamate. J Neurochem 90:683–693
Sousa SC, Castilho RF (2005) Protective effect of melatonin on rotenone plus Ca2+-induced mitochondrial oxidative stress and PC12 cell death. Antioxid Redox Signal 7:1110–1116
Berndt N, Holzhütter HG, Bulik S (2013) Implications of enzyme deficiencies on the mitochondrial energy metabolism and ROS formation of neurons involved in rotenone-induced Parkinson’s disease: A model-based analysis. FEBS J 280:5080–5093
Acknowledgments
This work was supported by grants from the São Paulo Research Foundation (FAPESP, #2011/50400-0) and the Brazilian National Council for Scientific and Technological Development (CNPq). L.G.B.M., C.E.B. and F.A.R. were supported by FAPESP (#2011/14229-4), CNPq and CAPES fellowships, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michelini, L.G.B., Benevento, C.E., Rossato, F.A. et al. Effects of Partial Inhibition of Respiratory Complex I on H2O2 Production by Isolated Brain Mitochondria in Different Respiratory States. Neurochem Res 39, 2419–2430 (2014). https://doi.org/10.1007/s11064-014-1446-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1446-4