Abstract
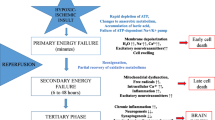
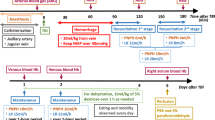
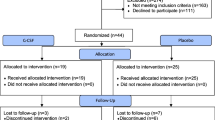
Ischemic brain injury continues to be of major concern in patients undergoing cardiopulmonary bypass (CPB) surgery for congenital heart disease. Striatum and hippocampus are particularly vulnerable to injury during these processes. Our hypothesis is that the neuronal injury resulting from CPB and the associated circulatory arrest can be at least partly ameliorated by pre-treatment with granulocyte colony stimulating factor (G-CSF). Fourteen male newborn piglets were assigned to three groups: deep hypothermic circulatory arrest (DHCA), DHCA with G-CSF, and sham-operated. The first two groups were placed on CPB, cooled to 18 °C, subjected to 60 min of DHCA, re-warmed and recovered for 8–9 h. At the end of experiment, the brains were perfused, fixed and cut into 10 µm transverse sections. Apoptotic cells were visualized by in situ DNA fragmentation assay (TUNEL), with the density of injured cells expressed as a mean number ± SD per mm2. The number of injured cells in the striatum and CA1 and CA3 regions of the hippocampus increased significantly following DHCA. In the striatum, the increase was from 0.46 ± 0.37 to 3.67 ± 1.57 (p = 0.002); in the CA1, from 0.11 ± 0.19 to 5.16 ± 1.57 (p = 0.001), and in the CA3, from 0.28 ± 0.25 to 2.98 ± 1.82 (p = 0.040). Injection of G-CSF prior to bypass significantly reduced the number of injured cells in the striatum and CA1 region, by 51 and 37 %, respectively. In the CA3 region, injured cell density did not differ between the G-CSF and control group. In a model of hypoxic brain insult associated with CPB, G-CSF significantly reduces neuronal injury in brain regions important for cognitive functions, suggesting it can significantly improve neurological outcomes from procedures requiring DHCA.


Similar content being viewed by others
References
Sheridan WP, Morstyn G, Wolf M et al (1989) Granulocyte colony-stimulating factor and neutrophil recovery after high-dose chemotherapy and autologous bone marrow transplantation. Lancet 2:891–895
Weaver CH, Buckner CD, Longin K et al (1993) Syngeneic transplantation with peripheral blood mononuclear cells collected after the administration of recombinant human granulocyte colony-stimulating factor. Blood 82:1981–1984
Kawabe J, Koda M, Hashimoto M et al (2011) Neuroprotective effects of granulocyte colony-stimulating factor and relationship to promotion of angiogenesis after spinal cord injury in rats: laboratory investigation. J Neurosurg Spine 15:414–421
Meuer K, Pitzer C, Teismann P et al (2006) Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson’s disease. J Neurochem 97:675–686
Tsai KJ, Tsai YC, Shen CK (2007) G-CSF rescues the memory impairment of animal models of Alzheimer’s disease. J Exp Med 204:1273–1280
Zhao LR, Navalitloha Y, Singhal S et al (2007) Hematopoietic growth factors pass through the blood-brain barrier in intact rats. Exp Neurol 204:569–573
Schneider A, Krüger C, Steigleder T et al (2005) The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest 115:2083–2098
Schäbitz WR, Kollmar R, Schwaninger M et al (2003) Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 34:745–751
Gibson CL, Bath PM, Murphy SP (2005) G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab 25:431–511
Shyu WC, Lin SZ, Yang HI et al (2004) Functional recovery of stroke rats induced by granulocyte colony-stimulating factor-stimulated stem cells. Circulation 110:1847–1854
Kawada H, Takizawa S, Takanashi T et al (2006) Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation 113:701–710
Hartung T (1998) Anti-inflammatory effects of granulocyte colony-stimulating factor. Curr Opin Hematol 5:221–225
Solaroglu I, Cahill J, Tsubokawa T et al (2009) Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res 31:167–172
Xiao BG, Lu CZ, Link H (2007) Cell biology and clinical promise of G-CSF: immunomodulation and neuroprotection. J Cell Mol Med 11:1272–1290
Pastuszko P, Schears GJ, Pirzadeh A et al (2012) Effect of granulocyte-colony stimulating factor (G-CSF) on expression of select proteins involved in apoptosis in a neonatal piglet brain following cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA). J Thorac Cardiovasc Surg 143:1436–1442
Pastuszko P, Schears GJ, Kubin J et al (2013) Granulocyte-colony stimulating factor suppresses early inflammatory response of striatum in a cardiopulmonary bypass-circulatory arrest model of ischemic brain injury in newborn piglets. World J Cardiovasc Dis 3:197–205
Zhao L-R, Navalitloha Y, Singhal S et al (2007) Hematopoietic growth factors pass through the brain-blood barrier in intact rats. Exp Neurol 204:569–573
Zhao R, Cui Q, Yu SQ et al (2009) Antegrade cerebral perfusion during deep hypothermia circulatory arrest attenuates the apoptosis of neurons in porcine hippocampus. Heart Surg Forum 12:E219–E224
Shuja F, Tabbara M, Li Y et al (2009) Profound hypothermia decreases cardiac apoptosis through Akt survival pathway. J Am Coll Surg 209:89–99
Shillingford AJ, Glanzman MM, Ittenbach RF et al (2008) Inattention, hyperactivity and school performance in a population of school age children with complex congenital heart disease. Pediatrics 121:e759–e767
Gaynor JW, Gerdes M, Nord AS et al (2010) Is cardiac diagnosis a predictor of neurodevelopmental outcome after cardiac surgery in infancy? J Thorac Cardiovasc Surg 140:1230–1237
Tabbutt S, Nord AS, Jarvik GP et al (2008) Neurodevelopmental outcomes after staged palliation for hypoplastic left heart syndrome. Pediatrics 121:476–483
Forbess JM, Visconti KJ, Hancock-Friesen C et al (2002) Neurodevelopmental outcome after congenital heart surgery: results from an institutional registry. Circulation 106:I95–I102
Bellinger DC, Jonas RA, Rappaport LA et al (1995) Developmental and neurologic status of children after heart surgery with hypothermic circulatory arrest or low-flow cardiopulmonary bypass. N Engl J Med 332:549–555
Ergin MA, Galla JD, Lansman L et al (1994) Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 107:788–797
Ferry PC (1990) Neurologic sequelae of open-heart surgery in children. An irritating question. Am J Dis Child 144:369–373
Mezrow CK, Gandsas A, Sadeghi AM et al (1995) Metabolic correlates of neurologic and behavioral injury after prolonged hypothermic circulatory arrest. J Thorac Cardiovasc Surg 109:959–975
Newman M, Frasco P, Kern F (1992) Central nervous system dysfunction after cardiac surgery. Adv Cardiovasc Surg 3:243–284
Newburger JW, Jonas RA, Wernovsky G et al (1993) A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med 329:1057–1064
Robinson M, Blumenthal JA, Burker EJ et al (1990) Coronary artery bypass grafting and cognitive function: a review. J Cardiopulm Rehab 10:180–189
Schell RM, Kern FH, Greeley WJ et al (1993) Cerebral blood flow and metabolism during cardiopulmonary bypass. Anesth Analg 76:849–865
Shaw PJ, Bates D, Cartlidge NE et al (1985) Early neurological complications of coronary artery bypass surgery. Br Med J 291:1384–1387
Hogue CW Jr, Sundt TM III, Goldberg M et al (1999) Neurological complications of cardiac surgery: the need for new paradigms in prevention and treatment. Semin Thorac Cardiovasc Surg 11:105–115
Taylor KM (1998) Central nervous system effects of cardiopulmonary bypass. Ann Thorac Surg 66:20–24
He XM, Mo XM, Gu Q et al (2010) Effect of diazoxide on oxygen free radicals and cell apoptosis in brain tissue after deep hypothermia cerebral ischemia reperfusion injury in young rats. Chin J Surg 48:142–145
Tseng EE, Brock MV, Lange MS et al (2010) Glutamate excitotoxicity mediates neuronal apoptosis after hypothermic circulatory arrest. Ann Thorac Surg 89:440–445
Zhao R, Cui Q, Yu SQ et al (2009) Antegrade cerebral perfusion during deep hypothermia circulatory arrest attenuates the apoptosis of neurons in porcine hippocampus. Heart Surg Forum 12:E219–E224
Chock VY, Amir G, Davis CR et al (2006) Antegrade cerebral perfusion reduces apoptotic neuronal injury in a neonatal piglet model of cardiopulmonary bypass. J Thorac Cardiovasc Surg 131:659–665
Pastuszko P, Pirzadeh A, Reade E et al (2009) The effect of hypothermia on neuronal viability following cardiopulmonary bypass and circulatory arrest in newborn piglets. Eur J Cardio Thorac Surg 35:577–581
Crain BJ, Westerkam WD, Harrison AH, Nadler JV (1988) Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience 27:387–402
Pulsinelli WA, Brierley JB, Plum F (1982) Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neural 11:491–498
Schmidt-Kastner R, Paschen W, Hossmann K-A (1991) Effect of transient global ischemia on the basal ganglia of rat. In: Bernardi G (ed) Basal ganglia, 3rd edn. Plenum Press, New York, pp 558–567
Wieloch T (1985) Neurochemical correlates to selective neuronal vulnerability. Prog Brain Res 63:69–85
Jackson TC, Rani A, Kumar A, Foster TC (2009) Regional hippocampal differences in AKT survival signaling across the lifespan: implications for CA1 vulnerability with aging. Cell Death Diff 16:439–448
Schmidt-Kastner R, Freund TF (1991) Selective vulnerability of the hippocampus in brain ischemia. Neuroscience 40:599–636
Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG (2007) Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci 27:4253–4260
De Jong GI, Farkas E, Stienstra CM et al (1999) Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience 91:203–210
Kreitzer AC (2009) Physiology and pharmacology of striatal neurons. Annu Rev Neurosci 32:127–147
Bird CM, Burgess N (2008) The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci 9:182–194
Solaroglu I, Tsubokawa T, Cahill J, Zhang JH (2006) Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience 143:965–974
Schäbitz WR, Kollmar R, Schwaninger M et al (2003) Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke 34:745–751
Dong F, Larner AC (2000) Activation of Akt kinase by granulocyte colony-stimulating factor (G-CSF): evidence for the role of a tyrosine kinase activity distinct from the Janus kinases. Blood 95:1656–1662
Cardone MH, Roy N, Stennicke HR et al (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321
del PesoL González-García M, Page C et al (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687–689
Dudek H, Datta SR, Franke TF et al (1997) Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661–665
Fujio Y, Guo K, Mano T et al (1999) Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol Cell Biol 19:5073–5082
Pugazhenthi S, Nesterova A, Sable C et al (2000) Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275:10761–10766
Monaghan DT, Cotman CW (1985) Distribution of N-methyl-o-aspartate-sensitive L-(3H)glutamate-binding sites in rat brain. J Neurosci 5:2909–2919
Jafari-Sabet M (2011) Involvement of dorsal hippocampal muscarinic cholinergic receptors on muscimol state-dependent memory of passive avoidance in mice. Life Sci 88:1136–1141
Watson DJ, Stanton ME (2009) Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weanling rats. Neurobiol Learn Mem 92:89–98
Pastuszko A (1994) Metabolic response of dopaminergic system during hypoxia-ischemia and reoxygenation in the immature brain. Rev Biochem Med Metabol Biol 51:1–15
Globus MY-T, Ginsberg MD, Dietrich WD et al (1987) Substantia nigra lesion protects against ischemic damage in the striatum. Neurosci Lett 80:251–256
Filloux F, Wamsley JK (1991) Dopaminergic modulation of excitotoxicity in the rat striatum: evidence from nigrostriatal lesion. Synapse 8:281–288
Globus MY-T, Busto R, Dietrich WD et al (1988) Intra-ischemic extracellular release of dopamine and glutamate is associated with striatal vulnerability to ischemia. Neurosci Lett 91:36–40
Hoyt KR, Reynolds IJ, Hastings TG (1997) Mechanisms of dopamine-induced cell death in cultured rat forebrain neurons: interactions with and differences from glutamate-induced cell death. Exp Neurol 143:269–281
Porat S, Simantov R (1999) Bcl-2 and p53: role in dopamine-induced apoptosis and differentiation. Ann NY Acad Sci 893:372–375
Noh JS, Kim EY, Kang JS et al (1999) Neurotoxic and neuroprotective actions of catecholamines in cortical neurons. Exp Neurol 159:217–224
Schultz S, Creed J, Schears G et al (2004) Comparison of low-flow cardiopulmonary bypass and circulatory arrest on brain oxygen and metabolism. Ann Thorac Surg 77:2138–2143
Marie C, Mossiat C, Beley A, Bralet J (1992) Alpha-methyl-para-tyrosine pretreatment protects from striatal neuronal death induced by four-vessel occlusion in the rat. Neurochem Res 17:961–965
Zaitseva T, Schears G, Schultz S et al (2005) Circulatory arrest and low-flow cardiopulmonary bypass alter CREB phosphorylation in piglet brain. Ann Thorac Surg 80:245–250
Zaitseva T, Shen J, Schears G et al (2002) Effect of catecholamines on activity of Na, K-ATPase in neonatal piglet brain during posthypoxic reoxygenation. Comp Biochem Physiol 132:139–145
Murphy S, Song D, Welsh FA et al (1996) The effect of catecholamines on regional expression of heat shock protein-72 mRNA in neonatal piglet brain during hypoxia and posthypoxic reoxygenation. Brain Res 727:145–152
Liew H-K, Hsu C-W, Kuo J-S, Pang C-Y (2012) Granulocyte-colony stimulating factor reduces striatal dopamine accumulation caused by cerebral ischemia. Tzu Chi Med J 24:e181–e185
Acknowledgments
The research was supported by a Grant HL-58669 from the National Institutes of Health, Bethesda, MD, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pastuszko, P., Schears, G.J., Greeley, W.J. et al. Granulocyte Colony Stimulating Factor Reduces Brain Injury in a Cardiopulmonary Bypass-Circulatory Arrest Model of Ischemia in a Newborn Piglet. Neurochem Res 39, 2085–2092 (2014). https://doi.org/10.1007/s11064-014-1399-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-014-1399-7