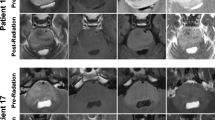
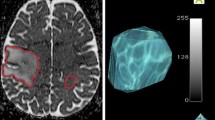
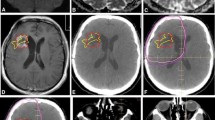
Abstract
Background and purpose
Baseline diffusion or apparent diffusion coefficient (ADC) characteristics have been shown to predict outcome related to DIPG, but the predictive value of post-radiation ADC is less well understood. ADC parametric mapping (FDM) was used to measure radiation-related changes in ADC and compared these metrics to baseline ADC in predicting progression-free survival and overall survival using a large multi-center cohort of DIPG patients (Pediatric Brain Tumor Consortium—PBTC).
Materials and methods
MR studies at baseline and post-RT in 95 DIPG patients were obtained and serial quantitative ADC parametric maps were generated from diffusion-weighted imaging based on T2/FLAIR and enhancement regions of interest (ROIs). Metrics assessed included total voxels with: increase in ADC (iADC); decrease in ADC (dADC), no change in ADC (nADC), fraction of voxels with increased ADC (fiADC), fraction of voxels with decreased ADC (fdADC), and the ratio of fiADC and fdADC (fDM Ratio).
Results
A total of 72 patients were included in the final analysis. Tumors with higher fiADC between baseline and the first RT time point showed a trend toward shorter PFS with a hazard ratio of 6.44 (CI 0.79, 52.79, p = 0.083). In contrast, tumors with higher log mean ADC at baseline had longer PFS, with a hazard ratio of 0.27 (CI 0.09, 0.82, p = 0.022). There was no significant association between fDM derived metrics and overall survival.
Conclusions
Baseline ADC values are a stronger predictor of outcome compared to radiation related ADC changes in pediatric DIPG. We show the feasibility of employing parametric mapping techniques in multi-center studies to quantitate spatially heterogeneous treatment response in pediatric tumors, including DIPG.


Similar content being viewed by others
Abbreviations
- fDM:
-
Functional diffusion map
- iADC:
-
Increase in ADC
- dADC:
-
Decrease in ADC
- nADC:
-
No change in ADC
- fiADC:
-
Fraction of voxels with iADC
- fdADC:
-
Fraction of voxels with dADC
- RT:
-
Radiation treatment
- OS:
-
Overall survival
- PFS:
-
Progression free survival
References
Pollack IF, Jakacki RI (2011) Childhood brain tumors: epidemiology, current management and future directions. Nat Rev Neurol 7(9):495–506. https://doi.org/10.1038/nrneurol.2011.110
Donaldson SS, Laningham F, Fisher PG (2006) Advances toward an understanding of brainstem gliomas. J Clin Oncol 24(8):1266–1272. https://doi.org/10.1200/JCO.2005.04.6599
Holodny AI, Makeyev S, Beattie BJ, Riad S, Blasberg RG (2010) Apparent diffusion coefficient of glial neoplasms: correlation with fluorodeoxyglucose-positron-emission tomography and gadolinium-enhanced MR imaging. AJNR Am J Neuroradiol 31(6):1042–1048. https://doi.org/10.3174/ajnr.A1989
Ellingson BM, Cloughesy TF, Zaw T et al (2012). Functional diffusion maps (fDMs) evaluated before and after radiochemotherapy predict progression-free and overall survival in newly diagnosed glioblastoma. Neuro Oncol 14(3):333–343
Löbel U, Sedlacik J, Reddick WE et al (2011) Quantitative diffusion-weighted and dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse. Intrinsic Pontine Glioma. https://doi.org/10.3174/ajnr.A2277
Poussaint TY, Vajapeyam S, Ricci KI et al (2015) Apparent diffusion coefficient histogram metrics correlate with survival in diffuse intrinsic pontine glioma: a report from the pediatric brain tumor consortium. Neuro Oncol 18(July):1–10. https://doi.org/10.1093/neuonc/nov256
Ellingson BM, Malkin MG, Rand SD et al (2010) Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 31(3):538–548
Moffat BA, Chenevert TL, Lawrence TS et al (2005) Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc Natl Acad Sci USA 102(15):5524–5529
Jain R, Ellika S, Scarpace L et al (2009) Role of functional diffusion maps as an imaging biomarker for treatment response assessment in recurrent/progressive malignant gliomas treated with bevacizumab. In: Proceedings 17th scientific meeting, international society for magnetic resonance in medicine, p 3471
Grech-Sollars M, Saunders DE, Phipps KP et al (2013). Challenges for the functional diffusion map in pediatric brain tumors. Neuro Oncol. https://doi.org/10.1093/neuonc/not197
Ceschin R, Kurland BF, Abberbock SR et al (2015) Parametric response mapping of apparent diffusion coefficient as an imaging biomarker to distinguish pseudoprogression from true tumor progression in peptide-based vaccine therapy for pediatric diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol. https://doi.org/10.3174/ajnr.A4428
Cohen KJ, Heideman RL, Zhou T et al (2011) Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol 13(4):410–416. https://doi.org/10.1093/neuonc/noq205
Ceschin R, Panigrahy A, Gopalakrishnan V. Sfdm (2015) Open-source software for temporal analysis and visualization of brain tumor diffusion mr using serial functional diffusion mapping. Cancer Inform 14:1–9. https://doi.org/10.4137/CIn.s17293
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL. Neuroimage 62(2):782–790
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29(3):162–173
Poussaint TY, Kocak M, Vajapeyam S et al (2011) MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC). Neuro Oncol 13(4):417–427. https://doi.org/10.1093/neuonc/noq200
Clerk-Lamalice O, Reddick WE, Li X et al (2016) MRI evaluation of non-necrotic T2-hyperintense foci in pediatric diffuse intrinsic pontine glioma. Am J Neuroradiol 37(10):1930–1937. https://doi.org/10.3174/ajnr.A4814
Chen HJ, Panigrahy A, Dhall G, Finlay JL, Nelson MD, Bluml S (2010) Apparent diffusion and fractional anisotropy of diffuse intrinsic brain stem gliomas. Am J Neuroradiol 31(10):1879–1885. https://doi.org/10.3174/ajnr.A2179
Zukotynski K, Vajapeyam S, Fahey FH et al (2017) Correlation of 18 F-FDG PET and MR apparent diffusion coefficient (ADC) histogram metrics with survival in diffuse intrinsic pontine glioma: a report from the pediatric brain tumor consortium. J Nucl Med. https://doi.org/10.2967/jnumed.116.185389
Lober RM, Cho Y-J, Tang Y et al (2014) Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. J Neurooncol 117(1):175–182. https://doi.org/10.1007/s11060-014-1375-8
Hipp SJ, Steffen-Smith E, Hammoud D, Shih JH, Bent R, Warren KE (2011) Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro Oncol 13(8):904–909. https://doi.org/10.1093/neuonc/nor076
Lapin DH, Tsoli M, Ziegler DS (2017) Genomic insights into diffuse intrinsic pontine glioma. Front Oncol 7:57. https://doi.org/10.3389/fonc.2017.00057
Diaz AK, Baker SJ (2014) The genetic signatures of pediatric high-grade glioma: no longer a one-act play. Semin Radiat Oncol 24(4):240–247. https://doi.org/10.1016/j.semradonc.2014.06.003
Conway AE, Reddick WE, Li Y et al (2014) “Occult” post-contrast signal enhancement in pediatric diffuse intrinsic pontine glioma is the MRI marker of angiogenesis? Neuroradiology 56(5):405–412. https://doi.org/10.1007/s00234-014-1348-9
Castel D, Philippe C, Calmon R et al (2015) Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol 130(6):815–827. https://doi.org/10.1007/s00401-015-1478-0
Hamisch C, Kickingereder P, Fischer M, Simon T, Ruge MI (2017) Update on the diagnostic value and safety of stereotactic biopsy for pediatric brainstem tumors: a systematic review and meta-analysis of 735 cases. J Neurosurg Pediatr 20(3):261–268. https://doi.org/10.3171/2017.2.PEDS1665
Funding
This study was funded by: National Institute of Health (NIH) [Grant No. U01 CA81457]; National Library of Medicine (NLM) Grant No. 5T15LM007059-27; Memorial Sloan Kettering Cancer Center (MSKCC) [Grant No. P30 CA008748]; The Pediatric Brain Tumor Consortium Foundation; the Pediatric Brain Tumor Foundation of the United States; the American Lebanese Syrian Associated Charities and Ian’s Friends Foundation.
Author information
Authors and Affiliations
Contributions
RC conceptualization, formal analysis, software, and writing—original draft, review and editing. MK statistical analysis, writing—review and editing. SV data curation, methodology, writing—review and editing. IFP project administration, writing—review and editing. AOT project administration, writing—review and editing. IJD writing—review and editing. TYP conceptualization, project administration, funding acquisition, writing—review and editing. AP conceptualization, project administration, funding acquisition, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Raf Ceschin and Dr. Ashok Panigrahy had full access to all of the data in the study and had final responsibility for the decision to submit for publication. The authors declare that there are no actual or potential conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ceschin, R., Kocak, M., Vajapeyam, S. et al. Quantifying radiation therapy response using apparent diffusion coefficient (ADC) parametric mapping of pediatric diffuse intrinsic pontine glioma: a report from the pediatric brain tumor consortium. J Neurooncol 143, 79–86 (2019). https://doi.org/10.1007/s11060-019-03133-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03133-y