Abstract
Introduction
The brain is a very soft tissue. Glioblastoma (GBM) brain tumours are highly infiltrative into the surrounding healthy brain tissue and invasion mechanisms that have been defined using rigid substrates therefore may not apply to GBM dissemination. GBMs characteristically lose expression of the high molecular weight tropomyosins, a class of actin-associating proteins and essential regulators of the actin stress fibres and focal adhesions that underpin cell migration on rigid substrates.
Methods
Here, we investigated how loss of the high molecular weight tropomyosins affects GBM on soft matrices that recapitulate the biomechanical architecture of the brain.
Results
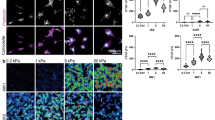
We find that Tpm 2.1 is down-regulated in GBM grown on soft substrates. We demonstrate that Tpm 2.1 depletion by siRNA induces cell spreading and elongation in soft 3D hydrogels, irrespective of matrix composition. Tpm 1.7, a second high molecular weight tropomyosin is also down-regulated when cells are cultured on soft brain-like surfaces and we show that effects of this isoform are matrix dependent, with Tpm 1.7 inducing cell rounding in 3D collagen gels. Finally, we show that the absence of Tpm 2.1 from primary patient-derived GBMs correlates with elongated, mesenchymal invasion.
Conclusions
We propose that Tpm 2.1 down-regulation facilitates GBM colonisation of the soft brain environment. This specialisation of the GBM actin cytoskeleton organisation that is highly suited to the soft brain-like environment may provide novel therapeutic targets for arresting GBM invasion.





Similar content being viewed by others
References
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21(8):1624–1636. https://doi.org/10.1200/jco.2003.05.063
Franze K (2013) The mechanical control of nervous system development. Development 140(15):3069–3077. https://doi.org/10.1242/dev.079145
Domingues HS, Cruz A, Chan JR, Relvas JB, Rubinstein B, Pinto IM (2018) Mechanical plasticity during oligodendrocyte differentiation and myelination. Glia 66(1):5–14. https://doi.org/10.1002/glia.23206
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689. https://doi.org/10.1016/j.cell.2006.06.044
Jagielska A, Norman AL, Whyte G, Vliet KJ, Guck J, Franklin RJ (2012) Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cell Dev 21(16):2905–2914. https://doi.org/10.1089/scd.2012.0189
Pogoda K, Janmey PA (2018) Glial tissue mechanics and mechanosensing by glial cells. Front Cell Neurosci 12:25. https://doi.org/10.3389/fncel.2018.00025
Pogoda K, Chin L, Georges PC, Byfield FJ, Bucki R, Kim R, Weaver M, Wells RG, Marcinkiewicz C, Janmey P (2014) Compression stiffening of brain and its effect on mechanosensing by glioma cells. New J Phys 16:075002. https://doi.org/10.1088/1367-2630/16/7/075002
De Clerck YA, Shimada H, Gonzalez-Gomez I, Raffel C (1994) Tumoral invasion in the central nervous system. J Neurooncol 18(2):111–121
Giese A, Westphal M (1996) Glioma invasion in the central nervous system. Neurosurgery 39(2):235–250 (discussion 250–232)
Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139(5):891–906. https://doi.org/10.1016/j.cell.2009.10.027
Kaufman LJ, Brangwynne CP, Kasza KE, Filippidi E, Gordon VD, Deisboeck TS, Weitz DA (2005) Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys J 89(1):635–650. https://doi.org/10.1529/biophysj.105.061994
Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P (2006) Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA 103(29):10889–10894
Soofi SS, Last JA, Liliensiek SJ, Nealey PF, Murphy CJ (2009) The elastic modulus of matrigel as determined by atomic force microscopy. J Struct Biol 167(3):216–219. https://doi.org/10.1016/j.jsb.2009.05.005
Wong SY, Ulrich TA, Deleyrolle LP, MacKay JL, Lin JM, Martuscello RT, Jundi MA, Reynolds BA, Kumar S (2015) Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. Cancer Res. https://doi.org/10.1158/0008-5472.can-13-3426
Ruiz-Ontanon P, Orgaz JL, Aldaz B, Elosegui-Artola A, Martino J, Berciano MT, Montero JA, Grande L, Nogueira L, Diaz-Moralli S, Esparis-Ogando A, Vazquez-Barquero A, Lafarga M, Pandiella A, Cascante M, Segura V, Martinez-Climent JA, Sanz-Moreno V, Fernandez-Luna JL (2013) Cellular plasticity confers migratory and invasive advantages to a population of glioblastoma-initiating cells that infiltrate peritumoral tissue. Stem Cell 31(6):1075–1085. https://doi.org/10.1002/stem.1349
Grundy TJ, De Leon E, Griffin KR, Stringer BW, Day BW, Fabry B, Cooper-White J, O’Neill GM (2016) Differential response of patient-derived primary glioblastoma cells to environmental stiffness. Sci Rep 6:23353. https://doi.org/10.1038/srep23353
Kim SN, Jeibmann A, Halama K, Witte HT, Walte M, Matzat T, Schillers H, Faber C, Senner V, Paulus W, Klambt C (2014) ECM stiffness regulates glial migration in Drosophila and mammalian glioma models. Development 141(16):3233–3242. https://doi.org/10.1242/dev.106039
Lee S, Kumar S (2016) Actomyosin stress fiber mechanosensing in 2D and 3D. F1000Research. https://doi.org/10.12688/f1000research.8800.1
Hughes JA, Cooke-Yarborough CM, Chadwick NC, Schevzov G, Arbuckle SM, Gunning P, Weinberger RP (2003) High-molecular-weight tropomyosins localize to the contractile rings of dividing CNS cells but are absent from malignant pediatric and adult CNS tumors. Glia 42(1):25–35
Tojkander S, Gateva G, Schevzov G, Hotulainen P, Naumanen P, Martin C, Gunning PW, Lappalainen P (2011) A molecular pathway for myosin II recruitment to stress fibers. Curr Biol 21(7):539–550. https://doi.org/10.1016/j.cub.2011.03.007
Bershadsky AD, Ballestrem C, Carramusa L, Zilberman Y, Gilquin B, Khochbin S, Alexandrova AY, Verkhovsky AB, Shemesh T, Kozlov MM (2006) Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J Cell Biol 85(3–4):165–173
Wolfenson H, Meacci G, Liu S, Stachowiak MR, Iskratsch T, Ghassemi S, Roca-Cusachs P, O’Shaughnessy B, Hone J, Sheetz MP (2016) Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat Cell Biol 18(1):33–42. https://doi.org/10.1038/ncb3277
Ulrich TA, de Juan Pardo EM, Kumar S (2009) The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69(10):4167–4174. https://doi.org/10.1158/0008-5472.can-08-4859
Tse JR, Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties. Curr Protoc Cell Biol 10:10. https://doi.org/10.1002/0471143030
Day BW, Stringer BW, Wilson J, Jeffree RL, Jamieson PR, Ensbey KS, Bruce ZC, Inglis P, Allan S, Winter C, Tollesson G, Campbell S, Lucas P, Findlay W, Kadrian D, Johnson D, Robertson T, Johns TG, Bartlett PF, Osborne GW, Boyd AW (2013) Glioma surgical aspirate: a viable source of tumor tissue for experimental research. Cancers 5(2):357–371. https://doi.org/10.3390/cancers5020357
Mitchell CB, O’Neill GM (2017) Rac GTPase regulation of 3D invasion in neuroblastomas lacking MYCN amplification. Cell Adhes Migr 11(1):68–79. https://doi.org/10.1080/19336918.2016.1183868
Yager ML, Hughes JA, Lovicu FJ, Gunning PW, Weinberger RP, O’Neill GM (2003) Functional analysis of the actin-binding protein, tropomyosin 1, in neuroblastoma. Br J Cancer 89(5):860–863
Schevzov G, Whittaker SP, Fath T, Lin JJ, Gunning PW (2011) Tropomyosin isoforms and reagents. Bioarchitecture 1(4):135–164. https://doi.org/10.4161/bioa.1.4.17897
Bradbury PM, Turner K, Mitchell C, Griffin KR, Middlemiss S, Lau L, Dagg R, Taran E, Cooper-White J, Fabry B, O’Neill GM (2017) The focal adhesion targeting (FAT) domain of p130 Crk associated substrate (p130Cas) confers mechanosensing function. J Cell Sci 130(7):1263–1273
Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135(3):510–523
Lees JG, Bach CTT, Bradbury P, Paul A, Gunning PW, O’Neill GM (2011) The actin-associating protein Tm5NM1 blocks mesenchymal motility without transition to amoeboid motility. Oncogene 30(10):1241–1251
Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, Strongin AY,, Friedl P, Br€“cker EB (2003) Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol 160(2):267–277
Gordon VD, Valentine MT, Gardel ML, Andor-Ardo D, Dennison S, Bogdanov AA, Weitz DA, Deisboeck TS (2003) Measuring the mechanical stress induced by an expanding multicellular tumor system: a case study. Exp Cell Res 289(1):58–66
O’Neill GM (2009) The coordination between actin filaments and adhesion in mesenchymal migration. Cell Adhes Migr 3(4):355–357
Bargon SD, Gunning PW, O’Neill GM (2005) The Cas family docking protein, HEF1, promotes the formation of neurite-like membrane extensions. Biochim Biophys Acta 1746(2):143–154
Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol 153(4):881–888
Stehn JR, Haass NK, Bonello T, Desouza M, Kottyan G, Treutlein H, Zeng J, Nascimento PR, Sequeira VB, Butler TL, Allanson M, Fath T, Hill TA, McCluskey A, Schevzov G, Palmer SJ, Hardeman EC, Winlaw D, Reeve VE, Dixon I, Weninger W, Cripe TP, Gunning PW (2013) A novel class of anticancer compounds targets the actin cytoskeleton in tumor cells. Cancer Res 73(16):5169–5182. https://doi.org/10.1158/0008-5472.can-12-4501
Currier MA, Stehn JR, Swain A, Chen D, Hook J, Eiffe E, Heaton A, Brown D, Nartker BA, Eaves DW, Kloss N, Treutlein H, Zeng J, Alieva IB, Dugina VB, Hardeman EC, Gunning PW, Cripe TP (2017) Identification of cancer-targeted tropomyosin inhibitors and their synergy with microtubule drugs. Mol Cancer Ther 16(8):1555–1565. https://doi.org/10.1158/1535-7163.mct-16-0873
Brayford S, Bryce NS, Schevzov G, Haynes EM, Bear JE, Hardeman EC, Gunning PW (2016) Tropomyosin promotes lamellipodial persistence by collaborating with Arp2/3 at the leading edge. Curr Biol 26(10):1312–1318. https://doi.org/10.1016/j.cub.2016.03.028
Acknowledgements
We thank Janine Woehlk, Dr Kylie Turner, Kaitlyn Griffin and Dr Peta Bradbury for technical assistance.
Funding
CM, BS, BD and GO are members of the Brain Cancer Discovery Collaborative, supported by the Cure Brain Cancer Foundation and the work was supported by a University of Sydney Strategic Priority Areas for Collaboration (SPARC) cancer grant to MB and GON.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Research involving human and animal participants
This article does not contain any studies with human participants.
Rights and permissions
About this article
Cite this article
Mitchell, C.B., Black, B., Sun, F. et al. Tropomyosin Tpm 2.1 loss induces glioblastoma spreading in soft brain-like environments. J Neurooncol 141, 303–313 (2019). https://doi.org/10.1007/s11060-018-03049-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03049-z