Abstract
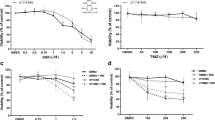
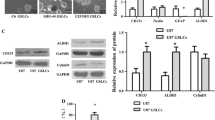
Glioblastoma (GBM) is the most aggressive and lethal form of brain cancer. Standard therapies are non-specific and often of limited effectiveness; thus, efforts are underway to uncover novel, unorthodox therapies against GBM. In previous studies, we investigated Withaferin A, a steroidal lactone from Ayurvedic medicine that inhibits proliferation in cancers including GBM. Another novel approach, tumor treating fields (TTFields), is thought to disrupt mitotic spindle formation and stymie proliferation of actively dividing cells. We hypothesized that combining TTFields with Withaferin A would synergistically inhibit proliferation in glioblastoma. Human glioblastoma cells (GBM2, GBM39, U87-MG) and human breast adenocarcinoma cells (MDA-MB-231) were isolated from primary tumors. The glioma cell lines were genetically engineered to express firefly luciferase. Proliferative potential was assessed either by bioluminescence imaging or cell counting via hemocytometer. TTFields (4 V/cm) significantly inhibited growth of the four cancer cell lines tested (n = 3 experiments per time point, four measurements per sample, p < 0.02 at least; 2-way ANOVA, control vs. treatment). The combination of Withaferin A (10–100 nM) with TTFields significantly inhibited the growth of the glioma cells to a degree beyond that of Withaferin A or TTFields alone. The interaction of the Withaferin A and TTFields on glioma cells was found to be synergistic in nature (p < 0.01, n = 3 experiments). These findings were validated by both bioluminescence and hemocytometric measurements. The combination of Withaferin A with TTFields represents a novel approach to treat GBM in a manner that is likely better than either treatment alone and that is synergistic.





Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BLI:
-
Bioluminescence imaging
- DAPI:
-
4′,6-Diamidino-2-Phenylindole
- DMEM:
-
Dulbecco’s modified eagle’s medium
- H-EGF:
-
Human epidermal growth factor
- EGFR:
-
Epidermal growth factor receptor
- EGFRvIII:
-
Epidermal growth factor receptor variant III
- FBS:
-
Fetal bovine serum
- FDA:
-
Food and drug administration
- H-FGF:
-
Human fibroblast growth factor
- GBM:
-
Glioblastoma
- GBM2:
-
Patient-derived glioblastoma cell culture (from Stanford University School of Medicine)
- GBM2/GFP-Luc:
-
GBM2 that was genetically modified to express a fusion protein of firefly luciferase and GFP
- GBM39:
-
Patient-derived glioblastoma cell culture (from University of California at San Diego School of Medicine)
- GBM39/Luc:
-
GBM39 that was genetically modified to express firefly luciferase
- GFP:
-
Green fluorescent protein
- GFP/Luc:
-
Fusion protein of GFP and firefly luciferase
- MDA-MB-231:
-
Human breast adenocarcinoma cancer cell line (from ATCC)
- PBS:
-
Phosphate-buffered saline
- H-PDGF-AA:
-
Human platelet-derived growth factor variant AA
- H-PDGF-BB:
-
Human platelet-derived growth factor variant BB
- TTField:
-
Tumor treating field
- U87-MG:
-
Human-derived GBM cell line that was purchased from ATCC
- U87-MG/eGFP-Luc:
-
U87-MG that was genetically modified to express a fusion protein of firefly luciferase and GFP
References
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, Hau P, Brandes AA, Gijtenbeek J, Marosi C, Vecht CJ, Mokhtari K, Wesseling P, Villa S, Eisenhauer E, Gorlia T, Weller M, Lacombe D, Cairncross JG, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups, National Cancer Institute of Canada Clinical Trials Group (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10: 459–466 doi:10.1016/S1470-2045(09)70025-7
Johnson DR, O’Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107:359–364. doi:10.1007/s11060-011-0749-4
Davies AM, Weinberg U, Palti Y (2013) Tumor treating fields: a new frontier in cancer therapy. Ann N Y Acad Sci 1291:86–95. doi:10.1111/nyas.12112
Giladi M, Schneiderman RS, Voloshin T, Porat Y, Munster M, Blat R, Sherbo S, Bomzon Z, Urman N, Itzhaki A, Cahal S, Shteingauz A, Chaudhry A, Kirson ED, Weinberg U, Palti Y (2015) Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep 5:18046 doi:10.1038/srep18046
Hottinger AF, Pacheco P, Stupp R (2016) Tumor treating fields: a novel treatment modality and its use in brain tumors. Neuro Oncol 18:1338–1349. doi:10.1093/neuonc/now182
Chaudhry A, Benson L, Varshaver M, Farber O, Weinberg U, Kirson E, Palti Y (2015) NovoTTF-100A System (Tumor treating fields) transducer array layout planning for glioblastoma: a NovoTAL system user study. World J Surg Oncol 13:316. doi:10.1186/s12957-015-0722-3
Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink KL, Barnett GH, Zhu JJ, Henson JW, Engelhard HH, Chen TC, Tran DD, Sroubek J, Tran ND, Hottinger AF, Landolfi J, Desai R, Caroli M, Kew Y, Honnorat J, Idbaih A, Kirson ED, Weinberg U, Palti Y, Hegi ME, Ram Z (2015) Maintenance therapy with tumor-treating fields plus Temozolomide vs Temozolomide alone for glioblastoma: a randomized clinical trial. JAMA 314:2535–2543. doi:10.1001/JAMA.2015.16669
Ansstas G, Tran DD (2016) Treatment with tumor-treating fields therapy and pulse dose bevacizumab in patients with bevacizumab-refractory recurrent glioblastoma: a case series. Case Rep Neurol 8:1–9 doi:10.1159/000442196
Fang HB, Ross DD, Sausville E, Tan M (2008) Experimental design and interaction analysis of combination studies of drugs with log-linear dose responses. Stat Med 27:3071–3083. doi:10.1002/sim.3204
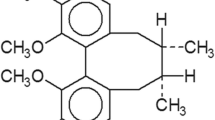
Lavie D, Glotter E, Shvo Y (1965) Constituents of Withania somnifera Dun. III. The side chain of Withaferin A. J Org Chem 30:1774–1778
Chang E, Pohling C, Natarajan A, Witney TH, Kaur J, Xu L, Gowrishankar G, A LDS, Murty S, Schick S, Chen L, Wu N, Khaw P, Mischel P, Abbasi T, Usmani S, Mallick P, Gambhir SS (2016) AshwaMAX and Withaferin A inhibits gliomas in cellular and murine orthotopic models. J Neurooncol 126:253–264. doi:10.1007/s11060-015-1972-1
Prasanna KS, Shilpa P, Salimath BP (2009) Withaferin A suppresses the expression of vascular endothelial growth factor in Ehrlich ascites tumor cells via Sp1 transcription factor. Curr Trends Biotechnol Pharm 3:138–148
Heyninck K, Lahtela-Kakkonen M, Van der Veken P, Haegeman G, Vanden Berghe W (2014) Withaferin A inhibits NF-kappaB activation by targeting cysteine 179 in IKKbeta. Biochem Pharmacol 91:501–509. doi:10.1016/j.bcp.2014.08.004
Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y, Liu B, Zhu Z, Zhang J (2013) MTA2 promotes gastric cancer cells invasion and is transcriptionally regulated by Sp1. Mol Cancer 12:102. doi:10.1186/1476-4598-12-102
Kustermans G, El Benna J, Piette J, Legrand-Poels S (2005) Perturbation of actin dynamics induces NF-kappaB activation in myelomonocytic cells through an NADPH oxidase-dependent pathway. Biochem J 387:531–540. doi:10.1042/BJ20041318
Nemeth ZH, Deitch EA, Davidson MT, Szabo C, Vizi ES, Hasko G (2004) Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J Cell Physiol 200:71–81. doi:10.1002/jcp.10477
Bargagna-Mohan P (2006) Small molecule anti-angiogenic probes of the ubiquitin proteasome pathway: potential application to choroidal neovascularization. Invest Ophthalmol Vis Sci 47:4138–4145. doi:10.1167/iovs.05-1452
Satelli A, Li S (2011) Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci 68:3033–3046. doi:10.1007/s00018-011-0735-1
Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby Ho Y, Mor-Vaknin N, Wendschlag N, Liu J, Evans RM, Markovitz DM, Zhan CG, Kim KB, Mohan R (2007) The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol 14:623–634. doi:10.1016/j.chembiol.2007.04.010
Grasso CS, Tang Y, Truffaux N, Berlow NE, Liu L, Debily MA, Quist MJ, Davis LE, Huang EC, Woo PJ, Ponnuswami A, Chen S, Johung TB, Sun W, Kogiso M, Du Y, Qi L, Huang Y, Hutt-Cabezas M, Warren KE, Le Dret L, Meltzer PS, Mao H, Quezado M, van Vuurden DG, Abraham J, Fouladi M, Svalina MN, Wang N, Hawkins C, Nazarian J, Alonso MM, Raabe EH, Hulleman E, Spellman PT, Li XN, Keller C, Pal R, Grill J, Monje M (2015) Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat Med. doi:10.1038/nm.3855
Cloughesy TF, Cavenee WK, Mischel PS (2014) Glioblastoma: from molecular pathology to targeted treatment. Annu Rev Pathol 9:1–25. doi:10.1146/annurev-pathol-011110-130324
Sarkaria JN, Yang L, Grogan PT, Kitange GJ, Carlson BL, Schroeder MA, Galanis E, Giannini C, Wu W, Dinca EB, James CD (2007) Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther 6:1167–1174. doi:10.1158/1535-7163.MCT-06-0691
Sasportas LS, Gambhir SS (2014) Imaging circulating tumor cells in freely moving awake small animals using a miniaturized intravital microscope. PLoS ONE 9:e86759. doi:10.1371/journal.pone.0086759
Slinker BK (1998) The statistics of synergism. J Mol Cell Cardiol 30:723–731. doi:10.1006/jmcc.1998.0655
Kirson ED, Schneiderman RS, Dbaly V, Tovarys F, Vymazal J, Itzhaki A, Mordechovich D, Gurvich Z, Shmueli E, Goldsher D, Wasserman Y, Palti Y (2009) Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC. Med Phys 9:1. doi:10.1186/1756-6649-9-1
Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y (2004) Disruption of cancer cell replication by alternating electric fields. Cancer Res 64:3288–3295
Clark PJ, Gaal J (2016) Effects of tumor treating fields (TTFields) and temozolomide in MGMT expressing and non-expressing patient-derived glioblastoma cells. Neuro Oncol 18:p. vi64
Groves M, Schneiderman R, Zeevi E, Voloshin T, Giladi M, Kirson E, Weinberg U (2016) Cytostatic agents combined with tumor treating fields (TTFields) in glioma cell lines. Neuro Oncol 18:p. vi133
Giladi M, Schneiderman RS, Porat Y, Munster M, Itzhaki A, Mordechovich D, Cahal S, Kirson ED, Weinberg U, Palti Y (2014) Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 14:54–63. doi:10.1016/j.pan.2013.11.009
Porat Y, Anna Shteingauz, Giladi M, Schneiderman R, Voloshin T, Munster M, Blat R, Kirson E, Weinberg U, Palti Y (2016) Tumor treating fields (TTFields) induce autophagy in glioma cells. Neuro Oncol 18:vi65–66
Kessler AF, Frömbling GE (2016) Tumor treating field (TTField) effects on glioblastoma cells are augumented by mitotic checkpoint inhibition. Neuro Oncol 18:p. vi61
O’Connell D, Shen V, Loudon W, Bota DA (2016) First report of tumor treating fields use in combination with bevacizumab in a pediatric patient: a case report. CNS Oncol. doi:10.2217/cns-2016-0018
Hottinger AF, Stupp R, Homicsko K (2014) Standards of care and novel approaches in the management of glioblastoma multiforme. Chin J Cancer 33:32–39. doi:10.5732/cjc.013.10207
Kim EH, Kim YJ, Song HS, Jeong YK, Lee JY, Sung J, Yoo SH, Yoon M (2016) Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. doi:10.18632/oncotarget.11407
Dhami J, Chang E, Gambhir SS (2016) Withaferin A and its potential role in glioblastoma (GBM). J Neurooncol. doi:10.1007/s11060-016-2303-x
Santagata S, Xu Y-m, Wijeratne EMK, Kontnik R, Rooney C, Perley CC, Kwon H, Clardy J, Kesari S, Whitesell L, Lindquist S, Gunatilaka AAL (2012) Using the heat-shock response to discover anticancer compounds that target protein homeostasis. ACS Chem Biol 7:340–349. doi:10.1021/cb200353m
Sharada AC, Solomon FE, Devi PU (2014) Toxicity of Withania somnifera root extract in rats and mice. Int J Pharmacogn 3:205–212
Devi PU (2014) Withania somnifera Dunal(Ashwagangha): potential plant source of a promising dug for cancer chemotherapy and radiosensitization. Indian J Exp Biol 34:927–932
Tiwari R, Chakraborty S, Saminathan M, Dhama K, Singh SV, al e (2014) Ashwagandha (Withania somnifera): role in safeguading health, immunomodulatory effects, combating infections and therapeutic application: a review. J Biol Sci 2:77–94
Raut A, Rege N, Shirolkar S, Pandey S, Tadvi F, Solanki P, Vaidya R, Vaidya A, Kene K (2012) Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integr Med 3:111. doi:10.4103/0975-9476.100168
Borasi G, Nahum A, Paulides MM, Powathil G, Russo G, Fariselli L, Lamia D, Cirincione R, Forte GI, Borrazzo C, Caccia B, di Castro E, Pozzi S, Gilardi MC (2016) Fast and high temperature hyperthermia coupled with radiotherapy as a possible new treatment for glioblastoma. J Ther Ultrasound 4:32. doi:10.1186/s40349-016-0078-3
Hodges TR, Ferguson SD, Heimberger AB (2016) Immunotherapy in glioblastoma: emerging options in precision medicine. CNS Oncol 5:175–186. doi:10.2217/cns-2016-0009
Gambhir L, Checker R, Sharma D, Thoh M, Patil A, Degani M, Gota V, Sandur SK (2015) Thiol dependent NF-kappaB suppression and inhibition of T-cell mediated adaptive immune responses by a naturally occurring steroidal lactone Withaferin A. Toxicol Appl Pharmacol 289:297–312. doi:10.1016/j.taap.2015.09.014
Diamant G, Ram Z, Volovitz I (2016) Evaluating the in-vitro effects of tumor-treating fields on T-cell responses. Neuro Oncol 18:p. vi93
Holtzman T (2016) Tumor treating fields exposure of tumor cells induce activation phenotype in immune cell. Neuro Oncol 18:p. vi92
Acknowledgements
We acknowledge the excellent technical support of Kathryn Li, Tara Thakurta, Alex Serafini and Xiaofan Wu. We gratefully acknowledge the Ben and Catherine Ivy Foundation (SSG) and the R25 Translational Neuroscience Training Grant R25NS065741 (CBP) for their support of our research. We thank Novocure Inc. for providing us with the inovitro™ device as well as technical support. Finally, we thank Dr. Moshe Giladi for all of his thoughts, comments and feedback.
Funding
Ben and Catherine Ivy Foundation (SSG) and the R25 Translational Neuroscience Training Grant R25NS065741 (CBP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest and agree on the submission of this manuscripts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chang, E., Pohling, C., Beygui, N. et al. Synergistic inhibition of glioma cell proliferation by Withaferin A and tumor treating fields. J Neurooncol 134, 259–268 (2017). https://doi.org/10.1007/s11060-017-2534-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2534-5