Abstract
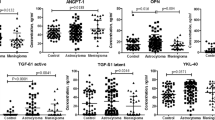
Serum amyloid A1 (SAA1) is a sensitive acute phase reactant primarily produced by the liver in response to acute inflammation. We have recently shown that SAA affects proliferation, migration, and invasion of glioblastoma cell lines, which suggest its participation in the malignant process. Consistently, levels of SAA have been used as a non-invasive biomarker for the prognosis of many cancers. In this study, we aimed to investigate SAA serum levels and expression of SAA genes in human astrocytomas tissues. Serum and tissue samples were obtained from patients with astrocytoma grades I to III and glioblastoma (GBM or grade IV). Levels of circulating SAA were significantly higher in the serum of patients with AGII-IV when compared to non-neoplastic samples derived from non-neoplastic patients (NN) (p > 0.0001). Quantitative real time PCR (qRT-PCR) of 148 astrocytomas samples (grades I-IV) showed that SAA1 mRNA was significantly higher in GBM when compared to AGI-III and NN samples (p < 0.0001). Immunohistochemistry analysis revealed cytoplasmic positivity for SAA in GBM. There was no correlation of SAA1 with clinical end-point of overall survival among GBM patients. However, it was found a positive correlation between SAA1 and genes involved in tumor progression, such as: HIF1A (r = 0.50; p < 0.00001), CD163 (r = 0.52; p < 0.00001), CXCR4 (r = 0.42; p < 0.00001) and CXCR7 (r = 0.33; p = 0.002). In conclusions, we show that astrocytoma patients have increased levels of serum SAA and SAA1 is expressed and secreted in GBM, and its co-expression with tumor-related genes supports its involvement in GBM angiogenesis and progression.




Similar content being viewed by others
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. doi:10.1007/s00401-007-0243-4
Chen J, Li Y, Yu TS, McKay RM, Burns DK, Kernie SG, Parada LF (2012) A restricted cell population propagates glioblastoma growth following chemotherapy. Nature 488:522–526. doi:10.1038/nature11287
LO HW (2010) Targeting Ras-RAF-ERK and its interactive pathways as a novel therapy for malignant gliomas. Curr Cancer Drug Targets 10:840–848. doi:10.2174/156800910793357970
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507. doi:10.1056/NEJMra0708126
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS (2013) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol 15:ii1–ii56. doi:10.1093/neuonc/not151
Ramankulov A, Lein M, Johannsen M, Schrader M, Miller K, Loening SA, Jung K (2008) Serum amyloid A as indicator of distant metastases but not as early tumor marker in patients with renal cell carcinoma. Cancer Lett 269:85–92. doi:10.1016/j.canlet.2008.04.022
Findeisen P, Zapatka M, Peccerella T, Matzk H, Neumaier M, Schadendorf D, Ugurel S (2009) Serum amyloid A as a prognostic marker in melanoma identified by proteomic profiling. J Clin Oncol 27:2199–2208. doi:10.1200/JCO.2008.18.0554
Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK (2013) Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst 105:1871–1880. doi:10.1093/jnci/djt309
Cho WC, Yip TT, Cheng WW, Au JS (2010) Serum amyloid A is elevated in the serum of lung cancer patients with poor prognosis. Br J Cancer 102:1731–1735. doi:10.1038/sj.bjc.6605700
Paret C, Schoen Z, Szponar A, Kovacs G (2010) Inflammatory protein serum amyloid A marks a subset of conventional renal cell carcinomas with fatal outcome. Eur Urol 57:859–866. doi:10.1016/j.eururo.2009.08.014
Chan DC, Chen CJ, Chu HC, Chang WK, Yu JC, Chen YJ, Wen LL, Huang SC, Ku CH, Liu YC, Chen JH (2007) Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol 14:84–93. doi:10.1245/s10434-006-9091-z
Liu C, Pan C, Shen J, Wang H, Yong L (2012) Identification of serum amyloid A in the serum of gastric cancer patients by protein expression profiling. Oncol Lett 3:1259–1262. doi:10.3892/ol.2012.664
Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, Gilliland FD, Sorensen BE, McTiernan A, Ulrich CM (2009) Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol 27:3437–3444. doi:10.1200/JCO.2008.18.9068
Menschikowski M, Hagelgans A, Fuessel S, Mareninova OA, Asatryan L, Wirth MP, Siegert G (2013) Serum amyloid A, phospholipase A(2)-IIA and C-reactive protein as inflammatory biomarkers for prostate diseases. Inflamm Res 62:1063–1072. doi:10.1007/s00011-013-0665-5
Cocco E, Bellone S, El-Sahwi K, Cargnelutti M, Buza N, Tavassoli FA, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD (2010) Serum amyloid A: a novel biomarker for endometrial cancer. Cancer 116:843–851. doi:10.1002/cncr.24838
Wang JY, Zheng YZ, Yang J, Lin YH, Dai SQ, Zhang G, Liu WL (2012) Elevated levels of serum amyloid A indicate poor prognosis in patients with esophageal squamous cell carcinoma. BMC Cancer 12:365. doi:10.1186/1471-2407-12-365
Kosari F, Parker AS, Kube DM, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Vasmatzis G (2005) Clear cell renal cell carcinoma: gene expression analyses identify a potential signature for tumor aggressiveness. Clin Cancer Res 11:5128–5139. doi:10.1158/1078-0432.CCR-05-0073
Khan N, Cromer CJ, Campa M, Patz EF Jr (2004) Clinical utility of serum amyloid A and macrophage migration inhibitory factor as serum biomarkers for the detection of non small cell lung carcinoma. Cancer 101:379–384. doi:10.1002/cncr.20377
Cho WC, Yip TT, Yip C, Yip V, Thulasiraman V, Ngan RK, Yip TT, Lau WH, Au JS, Law SC, Cheng WW, Ma VW, Lim CK (2004) Identification of serum amyloid a protein as a potentially useful biomarker to monitor relapse of nasopharyngeal cancer by serum proteomic profiling. Clin Cancer Res 10:43–52. doi:10.1158/1078-0432.CCR-0413-3
Cocco E, Bellone S, El-Sahwi K, Cargnelutti M, Casagrande F, Buza N, Tavassoli FA, Siegel ER, Visintin I, Ratner E, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD (2009) Serum amyloid A (SAA): a novel biomarker for uterine serous papillary cancer. Br J Cancer 101:335–341. doi:10.1038/sj.bjc.6605129
Yokoi K, Shih LC, Kobayashi R, Koomen J, Hawke D, Li D, Hamilton SR, Abbruzzese JL, Coombes KR, Fidler IJ (2005) Serum amyloid A as a tumor marker in sera of nude mice with orthotopic human pancreatic cancer and in plasma of patients with pancreatic cancer. Int J Oncol 27:1361–1369. doi:10.3892/ijo.27.5.1361
Weinstein PS, Skinner M, Sipe JD, Lokich JJ, Zamcheck N, Cohen AS (1984) Acute-phase proteins or tumour markers: the role of SAA, SAP, CRP and CEA as indicators of metastasis in a broad spectrum of neoplastic diseases. Scand J Immunol 19:193–198. doi:10.1111/j.1365-3083.1984.tb00919.x
Biran H, Friedman N, Neumann L, Pras M, Shainkin-Kestenbaum R (1986) Serum amyloid A (SAA) variations in patients with cancer: correlation with disease activity, stage, primary site, and prognosis. J Clin Pathol 39:794–797. doi:10.1136/jcp.39.7.794
Sasazuki S, Inoue M, Sawada N, Iwasaki M, Shimazu T, Yamaji T, Tsugane S (2010) Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan public health center-based prospective study. Carcinogenesis 31:712–718. doi:10.1093/carcin/bgq010
Ren Y, Wang H, Lu D, Xie X, Chen X, Peng J, Hu Q, Shi G, Liu S (2014) Expression of serum amyloid A in uterine cervical cancer. Diagn Pathol 21:9–16. doi:10.1186/1746-1596-9-16
Gutfeld O, Prus D, Ackerman Z, Dishon S, Linke RP, Levin M, Urieli-Shoval S (2006) Expression of serum amyloid A, in normal, dysplastic, and neoplastic human colonic mucosa: implication for a role in colonic tumorigenesis. J Histochem Cytochem 54:63–73. doi:10.1369/jhc.5A6645.2005
Michaeli A, Finci-Yeheskel Z, Dishon S, Linke RP, Levin M, Urieli-Shoval S (2008) Serum amyloid A enhances plasminogen activation: Implication for a role in colon cancer. Biochem Biophys Res Commun 368:368–373. doi:10.1016/j.bbrc.2008.01.079
Knebel FH, Albuquerque RC, Massaro RR, Maria-Engler SS, Campa A (2013) Dual effect of serum amyloid A on the invasiveness of glioma cells. Mediators Inflamm 2013:509089. doi:10.1155/2013/509089
Würth R, Bajetto A, Harrison JK, Barbieri F, Florio T (2014) CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front Cell Neurosci 8:144. doi:10.3389/fncel.2014.00144
De Oliveira EM, Sandri S, Knebel FH, Contesini CG, Campa A, Filippin-Monteiro FB (2013) Hypoxia increases serum amyloid A3 (SAA3) in differentiated 3T3-L1 adipocytes. Inflammation 36:1107–1110. doi:10.1007/s10753-013-9644-9
Marie SK, Okamoto OK, Uno M, Hasegawa AP, Oba-Shinjo SM, Cohen T, Camargo AA, Kosoy A, Carlotti CG Jr, Toledo S, Moreira-Filho CA, Zago MA, Simpson AJ, Caballero OL (2008) Maternal embryonic leucine zipper kinase transcript abundance correlates with malignancy grade in human astrocytomas. Int J Cancer 122:807–815. doi:10.1002/ijc.23189
Oba-Shinjo SM, Bengtson MH, Winnischofer SM, Colin C, Vedoy CG, de Mendonça Z, Marie SK, Sogayar MC (2005) Identification of novel differentially expressed genes in human astrocytomas by cDNA representational difference analysis. Mol Brain Res 140:25–33. doi:10.1016/j.molbrainres.2005.06.015
Bianco AM, Uno M, Oba-Shinjo SM, Clara CA, de Almeida Galatro TF, Rosemberg S, Teixeira MJ, Marie SKN (2015) CXCR7 and CXCR4 expressions in infiltrative astrocytomas and their interactions with HIF1α expression and IDH1 mutation. Pathol Oncol Res 21:229–240. doi:10.1007/s12253-014-9813-7
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108. doi:10.1038/nprot.2008.73
De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, Salio M, Middleton M, Cerundolo V (2010) Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol 11:1039–1046. doi:10.1038/ni.1942
Liang JS, Sloane JA, Wells JM, Abraham CR, Fine RE, Sipe JD (1997) Evidence for local production of acute phase response apolipoprotein serum amyloid A in Alzheimer’s disease brain. Neurosci Lett 225:73–76. doi:10.1016/S0304-3940(97)00196-1
Yu Y, Liu J, Li SQ, Peng L, Ye RD (2014) Serum amyloid A differentially activates microglia and astrocytes via the PI3K pathway. J Alzheimer’s Dis 38:133–144. doi:10.3233/JAD-130818
Moshkovskii SA (2012) Why do cancer cells produce serum amyloid A acute-phase protein? BioChemistry 77:339–341. doi:10.1134/S0006297912040037
Dubois LG, Campanati L, Righy C, D’Andrea-Meira I, Spohr TC, Porto-Carreiro I, Pereira CM, Balça-Silva J, Kahn SA, DosSantos MF, Oliveira MA, Ximenes-da-Silva A, Lopes MC, Faveret E, Gasparetto EL, Moura-Neto V (2014) Gliomas and the vascular fragility of the blood brain barrier. Front Cell Neurosci 8:418. doi:10.3389/fncel.2014.00418
Rankin EB, Giaccia AJ (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15:678–685. doi:10.1038/cdd.2008.21
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896. doi:10.1038/ni.1937
Wynn TA, Chawla A, Pollard JW (2013) Macrophage biology in development, homeostasis and disease. Nature 496:445–455. doi:10.1038/nature12034
Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008) The inflammatory microenvironment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66:1–9. doi:10.1016/j.critrevonc.2007.07.004
Ambarus CA, Krausz S, van Eijk M, Hamann J, Radstake TR, Reedquist KA, Tak PP, Baeten DL (2012) Systematic validation of specific phenotypic markers for in vitro polarized human macrophages. J Immunol Methods 375:196–206. doi:10.1016/j.jim.2011.10.013
Sun L, Zhou H, Zhu Z, Yan Q, Wang L, Liang Q, Ye RD (2015) Ex vivo and in vitro effect of serum amyloid a in the induction of macrophage M2 markers and efferocytosis of apoptotic neutrophils. J Immunol 194:4891–4900. doi:10.4049/jimmunol.1402164
Aiuti A, Tavian M, Cipponi A, Ficara F, Zappone E, Hoxie J, Peault B, Bordignon C (1999) Expression of cxcr4, the receptor for stromal cell-derived factor-1 on fetal and adult human lympho-hematopoietic progenitors. Eur J Immunol 29:1823–1831. doi:10.1002/(SICI)1521-4141(199906)29:06<1823::AID-IMMU1823>3.0.CO;2-B
Jankowski K, Kucia M, Wysoczynski M, Reca R, Zhao D, Trzyna E, Trent J, Peiper S, Zembala M, Ratajczak J, Houghton P, Janowska-Wieczorek A, Ratajczak MZ (2003) Both hepatocyte growth factor (hgf) and stromal-derived factor-1 regulate the metastatic behavior of human rhabdomyosarcoma cells, but only hgf enhances their resistance to radiochemotherapy. Cancer Res 63:7926–7935. ISSN: 0008–5472
Zhou Y, Larsen PH, Hao C, Yong VW (2002) Cxcr4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem 277:49481–49487. doi:10.1074/jbc.M206222200
Rempel SA, Dudas S, Ge S, Gutierrez JA (2000) Identification and localization of the cytokine SDF1 and its receptor, CXC chemokine receptor 4, to regions of necrosis and angiogenesis in human glioblastoma. Clin Cancer Res 6:102–111. ISSN: 1078–0432
Calatozzolo C, Canazza A, Pollo B, Di Pierro E, Ciusani E, Maderna E, Salce E, Sponza V, Frigerio S, Di Meco F, Schinelli S, Salmaggi A (2011) Expression of the new CXCL12 receptor, CXCR7, in gliomas. Cancer Biol Ther 11:242–253. doi:10.4161/cbt.11.2.13951
Acknowledgements
This work was supported by Grants from support: Sao Paulo Research Foundation (FAPESP, 2009/54187-9 and 2011/00469-3); National Counsel of Technological and Scientific Development (CNPq, Brasília); and Coordination of Improvement of Higher Education Personnel (CAPES, Brasília).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study has been approved by the Ethics Committee of Hospital das Clinicas of School of Medicine of University of São Paulo (691/05) and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Knebel, F.H., Uno, M., Galatro, T.F. et al. Serum amyloid A1 is upregulated in human glioblastoma. J Neurooncol 132, 383–391 (2017). https://doi.org/10.1007/s11060-017-2386-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-017-2386-z