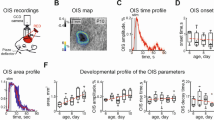
Recording of intrinsic optical signals (IOS) is widely used for functional mapping in the central nervous system. However, the effectiveness of the IOS method depends critically on the chromophore used. The most commonly used chromophores for detection of IOS in living organisms are the oxygenated and deoxygenated forms of hemoglobin, which provide for indirect assessment of neuron activity in terms of changes in oxygenated blood levels. However, in some cases these changes are weak, decreasing the effectiveness of the IOS method. We report here our studies of the potential for using water as the chromophore in the spectral recording of IOS. Stimulation of the sensory whiskers on the snouts of neonatal rat pups induced IOS in the corresponding parts of the animals’ sensorimotor cortex. Evoked IOS were recorded in the near infrared (IR) part of the spectrum. Shifts in the recorded spectral range were accompanied by decreases in the spontaneous noise level which, despite the low signal amplitude, led to a significant increase in the signal: noise ratio. These results provide evidence of the potential for using water as a marker for neural activity using the IOS recording method.
Similar content being viewed by others
References
Colonnese, M. and Khazipov, R., “Spontaneous activity in developing sensory circuits: Implications for resting state fMRI,” Neuroimage, 62, 2212–2221 (2012).
Colonnese, M. T., Phillips, M. A., Constantine-Paton, M., et al., “Development of hemodynamic responses and functional connectivity in rat somatosensory cortex,” Nat. Neurosci., 11, 72–79 (2008).
Grinvald, A., Lieke, E., Frostig, R. D., et al., “Functional architecture of cortex revealed by optical imaging of intrinsic signals,” Nature, 324, 361–364 (1986).
Hamann, S., Herrera-Perez, J. J., Zeuthen, T., and Alvarez-Leefmans, F. J., “Cotransport of water by the Na+–K+–2Cl(–) cotransporter NKCC1 in mammalian epithelial cells,” J. Physiol., 588, 4089–4101 (2010).
Hillman, E. M., “Optical brain imaging in vivo: techniques and applications from animal to man,” J. Biomed. Opt, 12, 051402 (2007).
Jourdain, P., Pavillon, N., Moratal, C., et al., “Determination of transmembrane water fluxes in neurons elicited by glutamate ionotropic receptors and by the cotransporters KCC2 and NKCC1: a digital holographic microscopy study,” J. Neurosci., 31, 11846–11854 (2011).
Kozberg, M. and Hillman, E., “Neurovascular coupling and energy metabolism in the developing brain,” Prog. Brain Res., 225, 213–242 (2016).
Layne, J., Yip, S., and Crook, R. B., “Down-regulation of Na–K–Cl cotransport by protein kinase C is mediated by protein phosphatase 1 in pigmented ciliary epithelial cells,” Exp. Eye Res., 72, 371–379 (2001).
MacAulay, N. and Zeuthen, T., “Water transport between CNS compartments: contributions of aquaporins and cotransporters,” Neuroscience, 168, 941–956 (2010).
MacVicar, B. A. and Hochman, D., “Imaging of synaptically evoked intrinsic optical signals in hippocampal slices,” J. Neurosci., 11, 1458–1469 (1991).
Minlebaev, M., Colonnese, M., Tsintsadze, T., et al., “Early gamma oscillations synchronize developing thalamus and cortex,” Science, 334, 226–229 (2011).
Mrsic-Flogel, T., Hubener, M., and Bonhoeffer, T., “Brain mapping: new wave optical imaging,” Curr. Biol., 13, R778–R780 (2003).
Nachabe, R., Evers, D. J., Hendriks, B. H., et al., “Effect of bile absorption coefficients on the estimation of liver tissue optical properties and related implications in discriminating healthy and tumorous samples,” Biomed. Opt. Express, 2, 600–614 (2011).
Sintsov, M., Suchkov, D., Khazipov, R., and Minlebaev, M., “Improved recordings of the optical intrinsic signals in the neonatal rat barrel cortex,” BioNanoScience (2016).
Tamura, M., Hoshi, Y., and Okada, F., “Localized near-infrared spectroscopy and functional optical imaging of brain activity,” Philos. Trans. R. Soc. Lond. B Biol. Sci., 352, 737–742 (1997).
Watanabe, E., Yamashita, Y., Maki, A., et al., “Non-invasive functional mapping with multi-channel near infra-red spectroscopic topography in humans,” Neurosci. Lett., 205, 41–44 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Vysshei Nervnoi Deyatel’nosti imeni I. P. Pavlova, Vol. 67, No. 5, pp. 94–100, September–October, 2017.
Rights and permissions
About this article
Cite this article
Sharipzyanova, L.S., Suchkov, D.S., Khazipov, R.N. et al. Local Changes in Water Balance as a Marker of Neuron Activity in the Somatosensory System in Neonatal Rat Pups. Neurosci Behav Physi 49, 222–226 (2019). https://doi.org/10.1007/s11055-019-00718-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-019-00718-y