Abstract
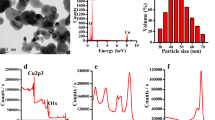
In view of increasing commercial applications of metal oxide nanoparticles their toxicity assessment becomes important. Alumina (Al2O3) nanoparticles have wide range of applications in industrial as well as personal care products. In the absence of prior report on toxicological impact of alumina nanoparticles to microalgae, the principal objective of this study was to demonstrate the effect of the nanoparticles on microalgae isolated from aquatic environment (Scenedesmus sp. and Chlorella sp.). The growth inhibitory effect of alumina nanoparticles was observed for both the species (72 h EC50 value, 45.4 mg/L for Chlorella sp.; 39.35 mg/L for Scenedesmus sp.). Bulk alumina also showed toxicity though to a lesser extent (72 h EC50 value, 110.2 mg/L for Chlorella sp.; 100.4 mg/L for Scenedesmus sp.). A clear decrease in chlorophyll content was observed in the treated cells compared to the untreated ones, more effect being notable in the case of nanoparticles. Preliminary results based on FT-IR studies, optical and scanning electron microscopic images suggest interaction of the nanoparticles with the cell surface.











Similar content being viewed by others
References
Chandradass J, Balasubramanian M (2006) Sol gel processing of alumina fibres. J Mater Process Technol 173:275–280
Chen KL, Elimelech M (2007) Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J Colloid Interface Sci 309(1):126–134
Driscoll CT, Schecher WD (1990) The chemistry of aluminium in the environment. Environ Geochem Health 12:28–48
Dumas P, Miller L (2003) The use of synchrotron infrared microspectroscopy in biological and biomedical investigations. Vib Spec 32:3–21
Fargasova A (2001) Interactive effect of manganese, molybdenum, nickel, copper I and II and vanadium on the freshwater alga Scenedesmus quadricauda. Bull Environ Contam Toxicol 67:688–695
Filella M, Buffle J (1993) Factors controlling the stability of submicron colloids in natural waters. Colloids Surf A: Physicochem Eng Aspects 73:255–273
Fujishima A, Rao TN, Tryk DA (2000) Titanium dioxide photocatalysis. J Photochem Photobiol C 1:1–21
Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS (2008) Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem 27(9):1972–1978
Guo J, Zhang X (2004) Metal-ion interactions with sugar. The crystal structure and FTIR study of an SrCl2-fructose complex. Carbohydr Res 339:1421–1426
Handy RD, Kammer FV, Lead JR, Hassellöv M, Owen R, Crane M (2008) The toxicology and chemistry of the manufactured nanoparticles. Ecotoxicology 17:287–314
Hoeckel V, DeSchamphelaere K, Vander Meeren KAC, Lucas P, Janssen SCR (2008) The ecotoxicity of silica nanoparticles to the alga Pseudokirchneriella subcapitata: importance of surface area. Environ Toxicol Chem 27:127–136
Huang CP, Cha DK, Ismat SS (2005) Progress report: short-term chronic toxicity of photocatalytic nanoparticles to bacteria, algae, and zooplankton. EPA Grant Number: R831721. http://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.abstractDetail/abstract/7384/report/0
Hund-Rinke K, Simon M (2006) Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ Sci Pollut Res 13:225–232
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano- and micro-scale oxide particles. Environ Pollut 157:1619–1625
Kaste PJ, Rice BM (2004) Novel energetic materials for the future force: the army pursues the next generation of propellant and explosives. AMPTIAC Q 8:84–90
Knox JP (1995) The extracellular-matrix in higher-plants. 4. Developmentally- regulated proteoglycans and glycoproteins of the plant-cell surface. J FASEB 9:1004–1012
Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdorster G, PhiLuria-bertani MA, Ryan J, Seaton A, Stone V, Tinkle SS, Tran L, Walker NJ, Warheit DB (2006) Safe handling of nanotechnology. Nature 444:267–269
Meng X, Dadachov M, Korfiatis GP, Christodoulatos C (2005) Methods of preparing a surface-activated titanium oxide product and of using same in water treatment processes. U.S. Patent Application Number 6,919,029
Naskar MK, Chatterjee M, Lakshmi NS (2002) Sol-emulsion-gel synthesis of hollow mullite microspheres. J Mater Sci 37:343–348
Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A, Quigg A, Santschi PH, Sigg L (2008) Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 17:372–386
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Oberdo¨rster G, Oberdörster E, Oberdörster J (2005) Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113:823–839
Organisation for Economic Cooperation and Development (1984) Algal growth inhibition test. OECD guidelines for testing of chemicals 201, Paris, France
Pena ME, Korfiatis GP, Patel M, Lippincott L, Meng X (2005) Adsorption of As(V) and As(III) by nanocrystalline titanium dioxide. Water Res 39:2327–2337
Sadiq MI, Chowdhury B, Chandrasekaran N, Mukherjee A (2009) Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomed Nanotechnol Biol Med 5:282–286
Saniger JM (1995) Al-O infrared vibrational frequencies of γ-A1203. Mater Lett 22:109–113
Tarte P (1967) Infra-red spectra of inorganic aluminates and characteristic vibrational frequencies of AlO4 tetrahedra and AlO6 octahedra. Spectrochim Acta 23A:2127–2143
The Royal Society & The Royal Academy of Engineering (2004) Nanoscience and nanotechnologies: opportunities and uncertainties. Royal Society Publications, London
U.S. Environmental Protection Agency Nanotechnology White Paper (2005) http://www.epa.gov/osa/pdfs/EPA_nanotechnology_white_paper_external_review_draft_12-02-2005.pdf
Yee N, Benning LG, Phoenix VR, Ferris FG (2004) Characterization of metal-Cyanobacteria sorption reactions: a combined Macroscopic and infrared spectroscopic investigation. Environ Sci Technol 38:775–782
Zhang WX (2003) Nanoscale iron particles for environmental remediation: an overview. J Nanopart Res 5:323–332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadiq, I.M., Pakrashi, S., Chandrasekaran, N. et al. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp.. J Nanopart Res 13, 3287–3299 (2011). https://doi.org/10.1007/s11051-011-0243-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-011-0243-0