Abstract
Background
The poor survival rate and undesirable homing of transplanted stem cells are the major challenges in stem cell therapy. Addressing the challenge would improve the therapeutic efficacy of these cells. Dimethyl fumarate (DMF) is an anti-inflammatory drug that exerts its effects through the activation of the nuclear factor erythroid 2–related factor 2 (Nrf2) pathway. Therefore, its cytoprotective effects on human adipose-derived MSCs (hASCs) against various oxidative stresses have been investigated in this study.
Methods and results
hASCs were cultured with different concentrations of DMF to evaluate the cytotoxicity of DMF on hASCs using Cell Counting Kit-8 (CCK-8). Besides, the migration ability of the cells after DMF treatment was evaluated using the Transwell method. Furthermore, the expression of HO-1 and NQO-1 was determined using RT-PCR. The cytoprotective effects of DMF on hASCs against the oxidative stress caused by H2O2 and Ultra Violet (UV) were evaluated by assessing cell proliferation and apoptosis. Our results demonstrated that under oxidative stress conditions induced by H2O2 and UV, DMF increased the survival rate and proliferation of the cells and prevented apoptosis. Moreover, the expression of HO-1 and NQO-1 was upregulated in hASCs pretreated with DMF which confirms the activation of the Nrf2 pathway. However, DMF significantly decreased migration in hADSCs (P < 0.0001).
Conclusion
Our findings indicate that DMF enhances the proliferation capability and viability of hASCs and prevents their apoptosis in harsh stressful microenvironments. However, the applicability of DMF as a cytoprotective factor for the augmentation of hASCs requires in-depth preclinical and clinical studies.





Similar content being viewed by others
Data availability
There is no data availability statement provided in the manuscript.
References
Alcayaga-Miranda F, Cuenca J, Khoury M (2017) Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol 8:339
Kalra K, Tomar P (2014) Stem cell: basics, classification and applications. Am J Phytomed Clin Ther 2(7):919–930
Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M (2015) Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther 6(1):199
Zhu S, Chen P, Wu Y, Xiong S, Sun H, Xia Q et al (2014) Programmed application of transforming growth factor β3 and Rac1 inhibitor NSC23766 committed hyaline cartilage differentiation of adipose-derived stem cells for osteochondral defect repair. Stem Cells Transl Med 3(10):1242–1251
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ et al (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7(2):211–228
Tao J, Wang H, Zhai Y, Park H, Wang J, Ji F et al (2016) Downregulation of Nrf2 promotes autophagy-dependent osteoblastic differentiation of adipose-derived mesenchymal stem cells. Exp Cell Res 349(2):221–229
Si Z, Wang X, Sun C, Kang Y, Xu J, Wang X et al (2019) Adipose-derived stem cells: sources, potency, and implications for regenerative therapies. Biomed Pharmacother 114:108765
Sobhani A, Khanlarkhani N, Baazm M, Mohammadzadeh F, Najafi A, Mehdinejadiani S et al (2017) Multipotent stem cell and current application. Acta Medica Iranica 55:6–23
Amiri F, Jahanian-Najafabadi A, Roudkenar MH (2015) In vitro augmentation of mesenchymal stem cells viability in stressful microenvironments. Cell Stress Chaperones 20(2):237–251
Zhou Y, Tsai T-L, Li W-J (2017) Strategies to retain properties of bone marrow–derived mesenchymal stem cells ex vivo. Ann N Y Acad Sci 1409(1):3
Gattazzo F, Urciuolo A (1840) Bonaldo P (2014) Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochimica et Biophysica Acta (BBA)-General Subjects 8:2506–2519
Mohammadzadeh M, Halabian R, Gharehbaghian A, Amirizadeh N, Jahanian-Najafabadi A, Roushandeh AM et al (2012) Nrf-2 overexpression in mesenchymal stem cells reduces oxidative stress-induced apoptosis and cytotoxicity. Cell Stress Chaperones 17(5):553–565
Zhu W, Chen J, Cong X, Hu S, Chen X (2006) Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem cells 24(2):416–425
Zhang D, Li Y, Zhu T, Zhang F, Yang Z, Miao D (2011) Zinc supplementation results in improved therapeutic potential of bone marrow-derived mesenchymal stromal cells in a mouse ischemic limb model. Cytotherapy 13(2):156–164
Zhu H, Zhang L, Itoh K, Yamamoto M, Ross D, Trush MA et al (2006) Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radical Biol Med 41(1):132–143
Surh Y-J, Kundu JK, Na H-K (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74(13):1526–1539
Motohashi H, Yamamoto M (2004) Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10(11):549–557
Silva-Islas CA, Maldonado PD (2018) Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol Res 134:92–99
Jing X, Shi H, Zhang C, Ren M, Han M, Wei X et al (2015) Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 286:131–140
Linker RA, Lee D-H, Ryan S, van Dam AM, Conrad R, Bista P et al (2011) Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain 134(3):678–692
Scannevin RH, Chollate S, Jung M-y, Shackett M, Patel H, Bista P et al (2012) Fumarates promote cytoprotection of central nervous system cells against oxidative stress via the nuclear factor (erythroid-derived 2)-like 2 pathway. J Pharmacol Exp Ther 341(1):274–284
Fu C-Y, Chen J, Lu X-Y, Zheng M-Z, Wang L-L, Shen Y-L et al (2019) Dimethyl fumarate attenuates lipopolysaccharide-induced mitochondrial injury by activating Nrf2 pathway in cardiomyocytes. Life Sci 235:116863
Garrido-Pascual P, Alonso-Varona A, Castro B, Burón M, Palomares T (2020) H 2 O 2-preconditioned human adipose-derived stem cells (HC016) increase their resistance to oxidative stress by overexpressing Nrf2 and bioenergetic adaptation. Stem Cell Res Ther 11(1):1–14
Abbasi-Malati Z, Roushandeh AM, Kuwahara Y, Roudkenar MH (2018) Mesenchymal stem cells on horizon: a new arsenal of therapeutic agents. Stem Cell Rev Rep 14(4):484–499
Hu C, Li L (2018) Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med 22(3):1428–1442
Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD (2002) Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105(1):93–98
Sart S, Ma T, Li Y (2014) Preconditioning stem cells for in vivo delivery. BioRes Open Access 3(4):137–149
Li Q, Wang Y, Deng Z (2013) Pre-conditioned mesenchymal stem cells: a better way for cell-based therapy. Stem Cell Res Ther 4(3):1–3
Bashiri H, Amiri F, Hosseini A, Hamidi M, Roushandeh AM, Kuwahara Y et al (2018) Dual preconditioning: a novel strategy to withstand mesenchymal stem cells against harsh microenvironments. Adv Pharm Bull 8(3):465
Li M, Yu H, Pan H, Xueqing Z, RUAN Q, Kong D et al (2019) Nrf2 suppression delays diabetic wound healing through sustained oxidative stress and inflammation. Front Pharmacol 10:1099
Liu X, Zhou W, Zhang X, Lu P, Du Q, Tao L et al (2016) Dimethyl fumarate ameliorates dextran sulfate sodium-induced murine experimental colitis by activating Nrf2 and suppressing NLRP3 inflammasome activation. Biochem Pharmacol 112:37–49
Robles L, Vaziri ND, Li S, Masuda Y, Takasu C, Takasu M et al (2014) Dimethyl fumarate protects pancreatic islet cells and non-endocrine tissue in L-arginine-induced chronic pancreatitis. PLoS ONE 9(9):e107111
Bayo Jimenez MT, Frenis K, Kröller-Schön S, Kuntic M, Stamm P, Kvandová M et al (2021) Noise-induced vascular dysfunction, oxidative stress, and inflammation are improved by pharmacological modulation of the NRF2/HO-1 axis. Antioxidants 10(4):625
Ibrahim SG, El-Emam SZ, Mohamed EA, Abd Ellah MF (2020) Dimethyl fumarate and curcumin attenuate hepatic ischemia/reperfusion injury via Nrf2/HO-1 activation and anti-inflammatory properties. Int Immunopharmacol 80:106131
Dwivedi DK, Jena G, Kumar V (2020) Dimethyl fumarate protects thioacetamide-induced liver damage in rats: studies on Nrf2, NLRP3, and NF-κB. J Biochem Mol Toxicol 34(6):e22476
Wang Q, Chuikov S, Taitano S, Wu Q, Rastogi A, Tuck SJ et al (2015) Dimethyl fumarate protects neural stem/progenitor cells and neurons from oxidative damage through Nrf2-ERK1/2 MAPK pathway. Int J Mol Sci 16(6):13885–13907
Lim JL, van der Pol SM, Di Dio F, van het Hof B, Kooij G, de Vries HE et al (2016) Protective effects of monomethyl fumarate at the inflamed blood–brain barrier. Microvasc Res 105:61–69
Kunze R, Urrutia A, Hoffmann A, Liu H, Helluy X, Pham M et al (2015) Dimethyl fumarate attenuates cerebral edema formation by protecting the blood–brain barrier integrity. Exp Neurol 266:99–111
Acknowledgements
The authors thank Research Deputy of Guilan University of Medical Sciences for their financial support.
Funding
This study has been supported by Guilan University of Medical Sciences (Grant Number: IR.GUMS.REC.1397.410).
Author information
Authors and Affiliations
Contributions
SS: Methodology, statistical analysis, preparation of manuscript draft. AMR: Conceptualization, Methodology, statistical analysis, Editing the manuscript, supervision. MHR: Conceptualization, Editing the manuscript. MHB: Conceptualization, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
There is no conflict interest among authors.
Ethical approval
All processes in this study were approved by ethic committee of Guilan University of Medical Sciences (Ethic approval number: IR.GUMS.REC.1397.410).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

11033_2021_6638_MOESM3_ESM.jpg
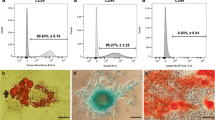
Supplementary file3 (JPG 310 kb) Supplementary Fig. 1 Characterization of adipose-derived mesenchymal stem cells detected by cells morphology, flowcytometry and their multilineage differentiation. (a) Morphology of the cells on day 4 (A), 7 (B), 11 (C) and 15 (D). (Scale bars=200 μm). (b) Flow cytometry for hASCs positive markers CD90, CD105 and CD73 (A, C, E), and hematopoietic markers, CD34 and CD45 (B and D). (F) Differentiation of hASCs to adipocytes (black arrows) and (G) shows the differentiation of the cells into osteocytes (white arrows). (Scale bar = 50µm) (Scale bar = 200 µm)

11033_2021_6638_MOESM4_ESM.jpg
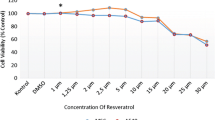
Supplementary file4 (JPG 337 kb) Supplementary Fig. 2 The cytotoxicity of Dimethyl fumarate (DMF) on hASCs in different concentrations and times. (a) Cell morphology after treatment of the cells with different concentrations of DMF for 24 hours (Scale bar = 50µm). (b) Cell viability and proliferation of hASCs were assessed after treatment of the cells with different concentrations of DMF (0, 5,10,20,30,40,50,60,70,80,90, and 100 μM) for 4, 12, 24 and 48 hours. (A-D) *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, ns; non-significant, N; triplicates
Rights and permissions
About this article
Cite this article
Shekarchi, S., Roushandeh, A.M., Roudkenar, M.H. et al. Dimethyl fumarate prevents cytotoxicity and apoptosis mediated by oxidative stress in human adipose-derived mesenchymal stem cells. Mol Biol Rep 48, 6375–6385 (2021). https://doi.org/10.1007/s11033-021-06638-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06638-w