Abstract
Pancreatic β cell damage is one of the crucial factors responsible for the development of type 2 diabetes mellitus (T2DM). Previous studies have suggested that puerarin (PR) could regulate the activities of the mitochondrial respiratory chain complex in diabetic nephropathy (DN); however, whether PR can inhibit pancreatic β-cell apoptosis in T2DM remains to be elucidated. In the present study, T2DM mice induced by high-fat diet and streptozotocin (STZ) injection were used as a working model to investigate the mechanism of PR on pancreatic β cell apoptosis. The results showed that PR decreased the serum fasting blood glucose (FBG), total cholesterol (TC), triglyceride (TG) and low-density lipoprotein (LDL) levels but significantly increased the fasting blood insulin (FINS) and high-density lipoprotein (HDL) levels. Furthermore, decreased caspase-3, 8, 9 and apoptosis-inducing factor (AIF) proteins in the pancreas were detected by Western blot analysis. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) staining demonstrated that the pancreatic β cell apoptosis was inhibited by PR. Furthermore, PR improved the histopathological changes in pancreatic tissue in T2DM mice. Collectively, the data show that PR can protect the β cells from apoptotic death in a mouse model of T2DM through regulating the expression of apoptosis-related protein-AIF and caspase family proteins.
Similar content being viewed by others
Introduction
T2DM is an endocrine disease that often accompanies other metabolic disorders, which are potentially life threatening [1]. Insulin resistance (IR) occurs in the early stage of T2DM and gradually leads to diminished insulin secretory ability of β cells due to structural damage that finally results in glucose and lipid metabolism disorders [2]. As is known, mitochondrial oxidative stress is a key factor contributing to IR and β cell dysfunction. Excess reactive oxygen species (ROS) could activate down-stream apoptotic factors including cytochrome C (Cyto-C) and AIF and induce β cell apoptosis [3, 4]. In our previous studies, we discovered that PR could decrease the FBG level of STZ-induced diabetic mice through ameliorating oxidative stress and reducing the expression of inflammatory factors nuclear-factor kappa B (NF-κB) and Cyto-C [5]. We therefore set out to explore the mechanism of PR on pancreas apoptosis in T2DM mice.
Traditionally, apoptosis is executed by the caspase protease family of proteins, which are activated through the exogenous death receptor pathway and endogenous mitochondrial pathway. The caspase family proteins increase the mitochondrial permeability, which then triggers Cyto-C release from the mitochondria and the formation of an apoptosis-inducing complex with Apaf-1, ATP and pro-caspase-9 [6, 7]. Previous studies have found that mitochondrial apoptosis-inducing factor (AIF) can mediate nuclear apoptosis [8, 9]. AIF is thought to play a central role in the caspase-independent apoptosis pathway. The pro-apoptotic effect of AIF is also reflected in its own positive feedback, that is, AIF released into the cytoplasm can act on other mitochondria, increasing their permeability and further promoting the release of AIF. AIF also promotes the release of Cyto-C, ultimately activating caspase-3. In our preliminary study, we discovered that PR could induce the activation of Bcl-2, a regulatory factor of AIF, which indicates that PR may inhibit apoptosis by regulating the expression of AIF. Therefore, we aimed to test this hypothesis to further elucidate the mechanism of PR on T2DM.
STZ is a classic drug to induce diabetes in mice. Mice fed a high fat diet and administered STZ show clinical manifestations of hyperglycaemia, hyperlipidaemia, obesity and hyperinsulinaemia [10]. Due to its instability, the STZ solution must be freshly prepared and directly injected into overnight fasted mice via the tail vein.
PR, one of the active compounds of Pueraria lobata, has been promoted as a therapy for DM through its role in elevating insulin expression and maintaining metabolic homoeostasis in STZ-induced diabetic mice. In the present study, we explore the effect of PR on T2DM. We demonstrate for the first time that PR could inhibit pancreatic cell apoptosis in T2DM mice through regulating the expression of caspase family proteins and AIF.
Materials and methods
Animals
All the animal protocols were approved by the Institutional Ethics Committee of Guangxi Medical University (Approval No. 2012011121). All the animal experiments are conducted in accordance with the guidelines of the Guiding Opinions on The Treatment of Experimental Animals for the care and use of laboratory animals. Healthy male Kunming mice weighing approximately 18–22 g were purchased from the Experimental Animal Center of Guangxi Medical University (Registration No. SCXK 2010-0002). The animals were acclimatized under temperature-controlled (22–25 °C) laboratory conditions with a 12 h light–dark cycle and were given free access to tap water and standard rodent chow.
Materials
A puerarin preparation (purity > 99%) was provided by the Department of Pharmaceutical Chemistry, Guangxi Medical University (Nanning, China). Metformin was purchased from Zhongxin Pharmaceutical Co., Ltd. (Tianjin, China). Streptozotocin (STZ) was obtained from Sigma Co., Ltd. (Missouri, USA). The molecular structure of PR is shown in Fig. 1. FBG and blood chemistry were measured with the Roche ACCU-CHEK® Performa (Strip lot: 470664, Switzerland) and an automatic biochemical analyser (Hitachi Model 7100 Automatic Analyzer), respectively. The other materials are outlined in the following sections.
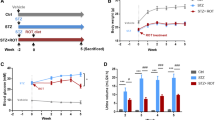
Experimental design [11]
Type 2 diabetic mice were established by high-fat diet feeding and STZ injection. Healthy male Kunming mice were fed a high-fat diet for 1 month and were intravenously injected with 80 mg kg−1 body weight STZ after a 12 h fast. The freshly-prepared STZ solution was dissolved in refrigerated saline in light-free. And metformin is dissolved directly in saline for oral gavage. Seventy-two hours later, FBG was measured, and the mice with FBG ≥ 11.1 mmol L−1 were considered as T2DM mice. In addition, healthy male Kunming mice fed a standard rodent chow served as normal controls. The experimental animals were divided into the following groups:
-
Group 1 healthy mice treated with a saline solution by gavage: normal control.
-
Group 2 Type 2 diabetic mice treated with a saline solution by gavage: model control.
-
Group 3 Type 2 diabetic mice treated with 320 mg kg−1 metformin by gavage: positive control.
-
Group 4 Type 2 diabetic mice treated with 80 mg kg−1 PR by gavage (our previous study found that 80 mg kg−1 of PR is an effective dose [5]).
Metformin and PR were given daily at the same time for up to 15 days.
Biochemical measurements
The FBG levels were detected during the experiment on day 0, 7 and 15 of treatment using the Roche ACCU-CHEK® Performa through tail vein blood. The serum samples were collected from whole blood by centrifugation at 1300×g for 10 min for detection. The serum FINS content was measured by the Cusabio mouse ELISA kits (Huamei Biotech Co., Ltd., Hubei, China). The serum levels of TC, TG, LDL and HDL were analysed using commercially available kits (Jiancheng Bioengineering Institute, Nanjing, China).
Pathological examination
An abdominal incision was performed to harvest the pancreas. Pancreatic tissues were fixed in 10% paraformaldehyde for 24 h and then embedded in paraffin. The sections (5 μm) were subjected to regular haematoxylin-eosin (HE) staining and observed under a light microscope Olympus CX4 (Japan).
Transmission electron microscopy
The pancreas tissues used for electron microscopy examination were removed under 0 °C, were cut into small pieces and immediately fixed in 2.5% pre-cooled glutaraldehyde. Ultrathin sections (70 nm) were subjected to uranylacetate and lead citrate staining. Finally, the samples were observed under a transmission electron microscopy (Hitachi H-7650).
Pancreatic β cell apoptosis assay
The in situ cell death detection kit (Roche, Germany) was applied to the pancreas tissue for terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) staining. The apoptotic cells were detected under the observation of a light microscope through the colour reaction.
Western blot analysis
The pancreas samples were homogenized in lysis buffer and total protein was extracted. The protein concentrations were determined using a protein assay reagent (Bio-Rad). For Western blot analysis, the protein lysates were resolved by SDS–polyacrylamide gel electrophoresis and transferred onto polyvinyldifluoride membranes. The membranes were blocked with PBST buffer (1% Tween-20, PBS) for 2 h at room temperature and then incubated with primary antibodies against caspase-3, 8, 9 and AIF (1:1000 Santa Cruz, USA) overnight at 4 °C. After three washes, the blots were incubated with a goat anti-rabbit and/or goat anti-mouse horseradish peroxidase-conjugated secondary IgG (Boster Biotechnology) for 2 h at room temperature. The immunoreactive bands were visualized with diaminobenzidine. The representative bands were measured by Scion image software (Scion Corp., Frederick, MD). The protein levels were normalized to those of β-actin.
Statistical analyses
The data are expressed as the mean ± S.E. The significant differences between the groups were analysed with one-way ANOVAs followed by Tukey’s tests for comparisons between groups using SPSS16.0. P-values < 0.05 were considered statistically significant.
Results
Effect of PR on serum levels of FBG and insulin in T2DM mice
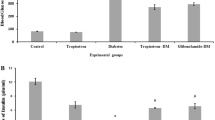
To investigate the hypoglycaemic effect of PR on T2DM mice, we administered 80 mg kg−1 PR orally for 15 days to high fat diet-fed and STZ-induced T2DM Kunming mice. As shown in Fig. 2 and Table 1, the oral administration of PR effectively decreased the FBG level relative to T2DM mice compared with the model control group, as well as the metformin-treated group. The FINS content significantly decreased in the T2DM mice administered metformin compared with the model controls. FINS were decreased in mice treated with PR showing difference (Fig. 3 and Table 1).
Effect of PR on serum lipid profiles in T2DM mice
Hyperglycaemia is often accompanied by hyperlipidaemia. Thus, we also measured the TC, TG, LDL and HDL levels to observe changes in lipid metabolism in T2DM mice. The serum levels of TC, TG and LDL were reduced by PR treatment compared to untreated diabetic mice. In addition, PR treatment enhanced the HDL level in diabetic mice compared with model controls (Fig. 4 and Table 2).
Effect of PR on morphological changes in the pancreas of T2DM mice
HE-stained pancreatic tissue showed that the cellular structure was maintained in healthy mice. Compared with the islets in normal samples, sparseness and cavitations were observed in the model control group due to the damage caused by STZ injection. The damage was alleviated by the administration of PR as shown in Fig. 5 and Table 3.
Pathophysiological observation of the pancreas (HE stain, n = 10, × 400). The pancreas samples were collected on the 15th day. a Normal control; b model control; c 320 mg kg−1 d−1 of metformin; d 80 mg kg−1 d−1 of PR. The results are presented as the mean ± S.E. *P < 0.05 compared with the model group; **P < 0.01 compared with the model group
Effect of PR on the pancreatic ultrastructure in T2DM mice
Ultrastructure observation provides a clear and direct view of organelles. As shown in Fig. 6 and Table 4, nuclear pyknosis and deformation features of apoptosis, as well as mitochondrial cavitation, were observed in the islet cells of diabetic mice. By contrast, T2DM mice treated with PR exhibited relatively fewer nuclear deformations and cavitated mitochondria.
Observation of pancreatic ultrastructure. The pancreas samples were collected on the 15th day. The red arrows point to the mitochondria in the pancreatic cells. a Normal control; b model control; c 320 mg kg−1 d−1 of metformin; d 80 mg kg−1 d−1 of PR. The results are presented as the mean ± S.E (n = 10). *P < 0.05 compared with the model group; **P < 0.01 compared with the model group
Effect of PR on pancreatic apoptosis in T2DM mice
TUNEL staining, in which positive cells appear brown, was conducted to investigate apoptosis in the pancreas. As shown in Fig. 7 and Table 5, the number of positive cells was greater in the T2DM mice compared to the normal controls. Treatment with PR as well as metformin significantly attenuated the decreased cell viability.
Observation of pancreatic apoptosis. The pancreas samples were collected on the 15th day. The red arrows point to the TUNEL-positive cells. a Normal control; b model control; c 320 mg kg−1 d−1 of metformin; d 80 mg kg−1 d−1 of PR. The results are presented as the mean ± S.E (n = 10). *P < 0.05 compared with the model group; **P < 0.01 compared with the model group
Effect of PR on the protein expressions of caspase-3, 8, 9 and AIF in T2DM mice
The caspase family of proteins is involved in inducing apoptosis. AIF is also a crucial factor responsible for mitochondrial apoptosis. Hence, we investigated whether PR affected the expression of caspase family proteins and AIF in T2DM mice. As shown in Fig. 8 and Table 6, increased caspase-3, 8, 9 and AIF in the pancreatic tissues from diabetic mice was observed. PR treatment effectively abolished the elevated protein expression levels of the caspase family proteins and AIF.
Discussion
Relative insulin deficiency is a well-known crucial factor in the progression of T2DM that contributes to the damage and apoptosis of islet β cells [12]. Thus, islet β cell repair is an efficient therapeutic target for T2DM treatment. In the present study, we demonstrated that PR can attenuate STZ-induced pancreatic cell apoptosis by inhibiting the expression of caspase family proteins and AIF.
PR, an isoflavonoid, is the main active component of P. lobata. PR has been reported to possess anti-oxidative, hypotensive and hypoglycaemic activities [13,14,15]. Prior studies have demonstrated that PR exerted protective effects on diabetic kidney injury, heart failure, diabetic cardiomyopathy, cardiac fibroblast proliferation, skeletal muscle energy metabolism and liver lipid metabolism [16,17,18,19,20,21,22]. The hypoglycaemic effect of PR on diabetes mellitus was confirmed by previous studies [15, 23, 24]. In our former study, PR treatment decreased the FBG level in diabetic nephropathy mice by attenuating oxidative stress. Another prior study found that PR is distributed in the pancreas, which may explain the hypoglycaemic manifestation of PR in diabetes [25].
High glucose-induced oxidative stress in diabetes would affect the regulation of insulin, which may lead to a reduction in insulin synthesis and promote β cell dysfunction. Mitochondria are damaged when exposed to excessive oxygen free radicals, which disturb the respiratory energy pathways [26]. Blood glucose control is the basis of DM treatment. In addition, β cell damage could be a therapeutic target. To detect the effect of PR on the pancreas, we examined the pancreatic ultrastructure and measured the expression of apoptosis-related proteins.
Previous research discovered two pathways that link mitochondrial and nuclear apoptosis: first, the AIF-dependent pathway, in which a large fragment of DNA breaks and peripheral chromosome agglutination occur upon AIF activation; second, the caspase-dependent pathway, where the production of oligonucleotide-like DNA fragments and further chromosome agglutination rely on the activation of Cyt-C, Apaf-1, caspase and CAD [26,27,28]. Caspase-3 is considered the most important protein in the caspase-dependent apoptosis pathway because it plays a key role in the initiation of apoptosis. Moreover, AIF is also thought to play a central role in the regulation of caspase-independent apoptosis. However, the investigation by Arnoult D reported that the apoptotic effect of AIF is dependent on caspase; study results showed that a caspase inhibitor had no effect on mitochondrial Cyt-C release, but AIF release was inhibited, and AIF exerted an apoptotic effect after the activation of caspase [29, 30]. Our WB results demonstrated that the expression of caspase-3, 8, 9 and AIF was reduced by PR, suggesting that PR prevents pancreatic cell apoptosis through inhibiting the caspase-dependent apoptotic pathway and AIF. These results corroborate our previous study showing that PR inhibited the expression of Cyt-C [5]. Together these data suggest that PR may prevent apoptosis by reducing the release of Cyt-C through regulating the caspase family proteins. Previous studies found that puerarin could inhibit the expression of caspase 3 in diabetic osteoblasts and glomerular epithelial cells, improving osteoporosis and glomerular fibrosis. Indicating that puerarin could inhibit apoptosis through regulating the caspase family [31, 32]. The results of present study are consistent with it; however, whether PR can inhibit both the caspase-dependent and caspase-independent AIF pathways remains to be further investigated.
The protective effect of PR on apoptosis was further confirmed by pathological and ultrastructure observations. PR treatment attenuated abnormal islet morphology including the shape and integrity of the cells. Specifically, nuclear deformation and mitochondrial cavitation were reduced following PR treatment. These effects may be due to the inhibition of caspase-3, 8, 9 and the AIF pathway; however, the AIF downstream signalling pathway still needs further verification.
Collectively, our findings provide new insights into the pathogenic process of pancreas injury and identify PR as a new therapy targeting the caspase/AIF/apoptosis pathway.
Change history
21 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11033-021-07112-3
Abbreviations
- T2DM:
-
Type 2 diabetes mellitus
- PR:
-
Puerarin
- STZ:
-
Streptozotocin
- FBG:
-
Fasting blood glucose
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
- LDL:
-
Low-density lipoprotein
- FINS:
-
Fasting blood insulin
- HDL:
-
High-density lipoprotein
- AIF:
-
Apoptosis-inducing factor
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling
- IR:
-
Insulin resistance
- ROS:
-
Reactive oxygen species
- Cyto-C:
-
Cytochrome C
- NF-κB:
-
Nuclear-factor kappa B
- HE:
-
Haematoxylin–eosin
References
Incani M, Sentinelli F, Perra L, Pani MG, Porcu M, Lenzi A, Cavallo MG, Cossu E, Leonetti F, Baroni MG (2015) Glycated hemoglobin for the diagnosis of diabetes and prediabetes: diagnostic impact on obese and lean subjects, and phenotypic characterization. J Diabetes Investig 6(1):44–50
Yang Y, Chan L (2016) Monogenic diabetes: what it teaches us on the common forms of type 1 and type 2 diabetes. Endocr Rev 37(3):190–222
Sodhi K, Maxwell K, Yan Y, Liu J, Chaudhry MA, Getty M, Xie Z, Abraham NG, Shapiro JI (2015) pNaKtide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci Adv 1(9):e1500781–e1500781
Bruder-Nascimento T, Silva MAD, Tostes RC (2014) The involvement of aldosterone on vascular insulin resistance: implications in obesity and type 2 diabetes. Diabetol Metab Syndr 6(1):1–8
Xu X, Ni Z, Chen Z, Huang W, Tao L, Hai K (2016) Puerarin, isolated from Pueraria lobata (Willd.), protects against diabetic nephropathy by attenuating oxidative stress. Gene 591(2):411–416
Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M (1999) Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397(6718):441–446
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HYM, Ravagnan L, Ferri KF (2001) Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410(6828):549–554
Alejandro S, Patricia E, Teresa G, Raúl M (2012) The 19 kDa Mycobacterium tuberculosis lipoprotein (LpqH) induces macrophage apoptosis through extrinsic and intrinsic pathways: a role for the mitochondrial apoptosis-inducing factor. Clin Dev Immunol 2012(2):950503
Yang S, Zhao X, Xu H, Chen F, Xu Y, Li Z, Sanchis D, Jin L, Zhang Y, Ye J (2017) AKT2 blocks nucleus translocation of apoptosis-inducing factor (AIF) and endonuclease G (EndoG) while promoting caspase activation during cardiac ischemia. Int J Mol Sci 18(3):565
Firouz D, Leiter EH, Guiming L, Jay R (2009) Animal models of diabetic uropathy. J Urol 182(6):S8–S13
Mali VR, Ruizhuo N, Jieli C, Xiao-Ping Y, Jiang X, Palaniyandi SS (2014) Impairment of aldehyde dehydrogenase-2 by 4-hydroxy-2-nonenal adduct formation and cardiomyocyte hypertrophy in mice fed a high-fat diet and injected with low-dose streptozotocin. Exp Biol Med 239(5):610–618
Chutima T, Shouhong X, Lin HV, Lori S, Domenico A (2012) Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell 150(6):1223–1234
Zhang J, Guo W, Tian B, Sun M, Li H, Zhou L, Liu X (2015) Puerarin attenuates cognitive dysfunction and oxidative stress in vascular dementia rats induced by chronic ischemia. Int J Clin Exp Pathol 8(11):4695–4704
Li W, Zhao W, Wu Q, Lu Y, Shi J, Chen X (2016) Puerarin improves diabetic aorta injury by inhibiting NADPH oxidase-derived oxidative stress in STZ-induced diabetic rats. J Diabetes Res 2016:8541520
Huang W, Jing W, Tao L, Liang M, Yan Y, Zeng X, Rui Z (2015) The protective effect of puerarin on myocardial infarction reperfusion injury (MIRI): a meta-analysis of randomized studies in rat models. Med Sci Monit Int Med J Exp Clin Res 21(1):1700–1706
Li X, Cai W, Lee K, Liu B, Deng Y, Chen Y, Zhang X, He JC, Zhong Y (2017) Publisher correction: puerarin attenuates diabetic kidney injury through the suppression of NOX4 expression in podocytes. Sci Rep 7(1):14603
Liu B, Zhao C, Li H, Chen X, Ding Y, Xu S (2018) Puerarin protects against heart failure induced by pressure overload through mitigation of ferroptosis. Biochem Biophys Res Commun 497(1):233–240
Gang CH, Shi-Fen PA, Xiang-Li CU, Li-Hong LI (2018) PuerarinattenuatesangiotensinII-inducedcardiacfibroblastproliferationviathepromotionofcatalaseactivityandtheinhibitionofhydrogenperoxide-dependentRac-1activation. Chin J Nat Med 16(1):41–52
Hu Y, Li X, Lin L, Liang S, Yan J (2018) Puerarin inhibits non-small cell lung cancer cell growth via the induction of apoptosis. Oncol Rep 39(4):1731–1738
Chen XF, Wang L, Wu YZ, Song SY, Min HY, Yang Y, He X, Liang Q, Yi L, Wang Y (2018) Effect of puerarin in promoting fatty acid oxidation by increasing mitochondrial oxidative capacity and biogenesis in skeletal muscle in diabetic rats. Nutr Diabetes 8(1):1
Yin MS, Zhang YC, Xu SH, Liu JJ, Sun XH, Liang C, Wang Y, Li J, Wang FW, Wang QL (2018) Puerarin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of inflammation. J Asian Nat Prod Res 21(5):1–18
Xing ZH, Yu-Chang MA, Xin-Ping LI, Zhang B, Zhang MD, Wan SM, Yang X, Yang TF, Jiang JW, Bao R (2017) Research progress of puerarin and its derivatives on anti-inflammatory and anti-gout activities. Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China J Chin Mater Med 42(19):3703
Pan X, Wang J, Pu Y, Yao J, Wang H (2015) Effect of puerarin on expression of ICAM-1 and TNF-α in kidneys of diabetic rats. Med Sci Monit Int Med J Exp Clin Res 21(64):2134–2140
Zheng G, Lin L, Zhong S, Zhang Q, Li D (2015) Effects of puerarin on lipid accumulation and metabolism in high-fat diet-fed mice. PLoS ONE 10(3):e0122925
Prasain JK, Peng N, Moore R, Arabshahi A, Barnes S, Wyss JM (2009) Tissue distribution of puerarin and its conjugated metabolites in rats assessed by liquid chromatography–tandem mass spectrometry. Phytomedicine 16(1):65–71
Ling-Yuan XU, Rong LI, Liang T, Duan XQ, Wei SJ (2013) Experimental study of reversion of puerariae radix lobatae flavonoids on pancreas injury induced by streptozotocin in mice. Chin J Exp Tradit Med Formul 19(8):231–234
Loeffler M, Daugas E, Susin SA, Zamzami N, Metivier D, Nieminen AL, Brothers G, Penninger JM, Kroemer G (2001) Dominant cell death induction by extramitochondrially targeted apoptosis-inducing factor. FASEB J 15(3):758–767
Daugas E, Susin SA, Zamzami N, Ferri KF, Irinopoulou T, Larochette N, Prévost MC, Leber B, Andrews D, Penninger J (2000) Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J Off Publ Fed Am Soc Exp Biol 14(5):729
Damien A, Philippe P, Jean-Claude M, Bruno A, Jerome E, Jean Claude A (2002) Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol 159(6):923
Damien A, Brigitte G, Mariusz K, Sharpe JC, Francesco C, Youle RJ (2014) Mitochondrial release of AIF and EndoG requires caspase activation downstream of Bax/Bak-mediated permeabilization. EMBO J 22(17):4385–4399
Liang J, Chen H, Pan W, Xu C (2012) Puerarin inhibits caspase-3 expression in osteoblasts of diabetic rats. Mol Med Rep 5(6):1419–1422
Zhou X, Bai C, Sun X, Gong X, Yang Y, Chen C, Shan G, Yao Q (2017) Puerarin attenuates renal fibrosis by reducing oxidative stress induced-epithelial cell apoptosis via MAPK signal pathways in vivo and in vitro. Ren Fail 39(1):423–431
Acknowledgements
This work was supported by the Guangxi Natural Science Foundation (Grant Nos. 2018GXNSFBA281037, 2018GXNSFBA281096); the Youth Science Foundation of Guangxi Medical University (Grant No. GXMUYSF201635); and the Guangxi Middle School and Young Teachers’ Basic Ability Improvement Project (Grant No. 2018KY0128).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liang, T., Xu, X., Ye, D. et al. Caspase/AIF/apoptosis pathway: a new target of puerarin for diabetes mellitus therapy. Mol Biol Rep 46, 4787–4797 (2019). https://doi.org/10.1007/s11033-019-04925-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04925-1