Abstract
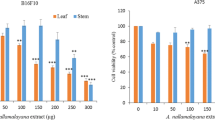
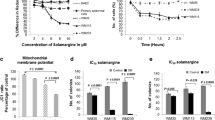
Cutaneous melanoma (CM) is an extremely aggressive cancer presenting low survival and high mortality. The vast majority of patients affected by this disease does not respond or show resistance to the chemotherapeutic drugs, which makes the treatment ineffective. In this sense, the necessity for the development of new agents to assist in CM therapy is extremely important. One of the sources of great interest in this search are compounds of natural origin. Among these compounds, caffeic acid has demonstrated a broad spectrum of pharmacological activities as well as antitumor effects in some types of cancer. Therefore, the objective of this work was to investigate the possible antitumor effect of caffeic acid on the SK-Mel-28 cell line, human CM cells. Cells were cultured in flasks with culture medium containing fetal bovine serum, antibiotic, and antifungal, and maintained in ideal conditions. Cells were treated with 25 µM, 50 µM, 100 µM, 150 µM and 200 µM of caffeic acid and dacarbazine at 1 mg/mL. We verified the effect on cell viability and cell death, apoptosis, cell cycle, colony formation and gene expression of caspases. Results showed a decrease in cell viability, cell death induction by apoptosis, inhibition of colony formation, modulation of cell cycle and alterations in gene expression of caspases after caffeic acid treatment. These results suggest an antitumor effect of the compound on SK-Mel-28 cells. This study provides original information on mechanisms by which caffeic acid may play a key role in preventing tumor progression in human melanoma cells.



Similar content being viewed by others
References
O’Sullivan J, O’Connor D (2018) The modern approach to targeting melanoma. In: Human skin cancers—pathways, mechanisms, targets and treatments. InTechOpen, London
Akbani R, Akdemir KC, Aksoy BA et al (2015) Genomic classification of cutaneous melanoma. Cell 161:1681–1696
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM (2011) Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16:5–24
Gogas HJ, Kirkwood JM, Sondak VK (2007) Chemotherapy for metastatic melanoma: time for a change? Cancer 109:455–464
Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates A, Dreno B, Henz M, Schadendorf D, Kapp A, Weiss J, Fraass U, Statkevich P, Muller M, Thatcher N (2000) Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 18:158–166
Sanlorenzo M, Vujic I, Posch C, Dajee A, Yen A, Kim S, Ashworth M, Rosenblum MD, Algazi A, Osella-Abate S, Quaglino P, Daud A, Ortiz-Urda S (2014) Melanoma immunotherapy. Cancer Biol Ther 15:665–674
Franklin C, Livingstone E, Roesch A, Schilling B, Schadendorf D (2017) Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol 43:604–611
Tapiero H, Tew K, Nguyen Ba G, Mathé G (2002) Polyphenols: do they play a role in the prevention of human pathologies? Biomed Pharmacother 56:200–207
Shi J, Yu J, Pohorly JE, Kakuda Y (2003) Polyphenolics in grape seeds-biochemistry and functionality. J Med Food 6:291–299
Weng CJ, Yen GC (2012) Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev 38:76–87
Jaganathan SK (2012) Growth inhibition by caffeic acid, one of the phenolic constituents of honey, in HCT 15 colon cancer cells. Sci World J 2012:372345
Assmann CE, Cadoná FC, Bonadiman BDSR, Dornelles EB, Trevisan G, Cruz IBMD (2018) Tea tree oil presents in vitro antitumor activity on breast cancer cells without cytotoxic effects on fibroblasts and on peripheral blood mononuclear cells. Biomed Pharmacother 103:1253–1261
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Konopka K, Pretzer E, Felgner PL, Düzgüneş N (1996) Human immunodeficiency virus type-1 (HIV-1) infection increases the sensitivity of macrophages and THP-1 cells to cytotoxicity by cationic liposomes. Biochim Biophys Acta 1312:186–196
Vermes I, Haanen C, Steffens-Nakken H, Reutellingsperger C (1995) A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods 184:39–51
da Silva LM, Frión-Herrera Y, Bartolomeu AR, Gorgulho CM, Sforcin JM (2017) Mechanisms involved in the cytotoxic action of Brazilian propolis and caffeic acid against HEp-2 cells and modulation of P-glycoprotein activity. J Pharm Pharmacol 69:1625–1633
Cubillos-Rojas M, Amair-Pinedo F, Peiró-Jordán R, Bartrons R, Ventura F, Rosa JL (2014) The E3 ubiquitin protein ligase HERC2 modulates the activity of tumor protein p53 by regulating its oligomerization. J Biol Chem 289:14782–14795
Kampa M, Alexaki V, Notas G, Nifli AP, Nistikaki A, Hatzoglou A, Bakogeorgou E, Kouimtzoglou E, Blekas G, Boskou D, Gravanis A, Castanas E (2004) Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: potential mechanisms of action. Breast Cancer Res 6:R63–R74
Takahashi H, Nguyen BCQ, Uto Y, Shahinozzaman M, Tawata S, Maruta H (2017) 1,2,3-Triazolyl esterization of PAK1-blocking propolis ingredients, artepillin C (ARC) and caffeic acid (CA), for boosting their anti-cancer/anti-PAK1 activities along with cell-permeability. Drug Discov Ther 11:104–109
Chang WC, Hsieh CH, Hsiao MW, Lin WC, Hung YC, Ye JC (2010) Caffeic acid induces apoptosis in human cervical cancer cells through the mitochondrial pathway. Taiwan J Obstet Gynecol 49:419–424
Huang W, Seo J, Willingham SB, Czyzewski AM, Gonzalgo ML, Weissman IL, Barron AE (2014) Learning from host-defense peptides: cationic, amphipathic peptoids with potent anticancer activity. PLoS ONE 9:e90397
Mulder KC, Lima LA, Miranda VJ, Dias SC, Franco OL (2013) Current scenario of peptide-based drugs: the key roles of cationic antitumor and antiviral peptides. Front Microbiol 4:321
Búfalo MC, Sforcin JM (2015) The modulatory effects of caffeic acid on human monocytes and its involvement in propolis action. J Pharm Pharmacol 67:740–745
Kilani-Jaziri S, Mokdad-Bzeouich I, Krifa M, Nasr N, Ghedira K, Chekir-Ghedira L (2017) Immunomodulatory and cellular anti-oxidant activities of caffeic, ferulic, and p-coumaric phenolic acids: a structure-activity relationship study. Drug Chem Toxicol 40:416–424
Murad LD, Soares Nda C, Brand C, Monteiro MC, Teodoro AJ (2015) Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr Cancer 67:532–542
Chen YN (2017) Dacarbazine inhibits proliferation of melanoma FEMX-1 cells by up-regulating expression of miRNA-200. Eur Rev Med Pharmacol Sci 21:1191–1197
Baharara J, Amini E, Nikdel N, Salek-Abdollahi F (2016) The cytotoxicity of dacarbazine potentiated by sea cucumber saponin in resistant B16F10 melanoma cells through apoptosis induction. Avicenna J Med Biotechnol 8:112–119
He J, Xu Q, Jing Y, Agani F, Qian X, Carpenter R, Li Q, Wang XR, Peiper SS, Lu Z, Liu LZ, Jiang BH (2012) Reactive oxygen species regulate ERBB2 and ERBB3 expression via miR-199a/125b and DNA methylation. EMBO Rep 13:1116–1122
Grabsch H, Takeno S, Parsons WJ, Pomjanski N, Boecking A, Gabbert HE, Mueller W (2003) Overexpression of the mitotic checkpoint genes BUB1, BUBR1, and BUB3 in gastric cancer-association with tumour cell proliferation. J Pathol 200:16–22
Pinto M, Vieira J, Ribeiro FR, Soares MJ, Henrique R, Oliveira J, Jerónimo C, Teixeira MR (2008) Overexpression of the mitotic checkpoint genes BUB1 and BUBR1 is associated with genomic complexity in clear cell kidney carcinomas. Cell Oncol 30:389–395
Burum-Auensen E, DeAngelis PM, Schjølberg AR, Røislien J, Mjåland O, Clausen OP (2008) Reduced level of the spindle checkpoint protein BUB1B is associated with aneuploidy in colorectal cancers. Cell Prolif 41:645–659
Kato T, Daigo Y, Aragaki M, Ishikawa K, Sato M, Kondo S, Kaji M (2011) Overexpression of MAD2 predicts clinical outcome in primary lung cancer patients. Lung Cancer 74:124–131
Min J, Shen H, Xi W, Wang Q, Yin L, Zhang Y, Yu Y, Yang Q, Wang ZN (2018) Synergistic anticancer activity of combined use of caffeic acid with paclitaxel enhances apoptosis of non-small-cell lung cancer H1299 cells in vivo and in vitro. Cell Physiol Biochem 48:1433–1442
Dziedzic A, Kubina R, Kabała-Dzik A, Tanasiewicz M (2017) Induction of cell cycle arrest and apoptotic response of head and neck squamous carcinoma cells (Detroit 562) by caffeic acid and caffeic acid phenethyl ester derivative. Evid Based Complement Altern Med 2017:6793456
Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, Jastrzębska-Stojko Ż, Stojko R, Wojtyczka RD, Stojko J (2017) Comparison of two components of propolis: caffeic acid (CA) and caffeic acid phenethyl ester (CAPE) induce apoptosis and cell cycle arrest of breast cancer cells MDA-MB-231. Molecules 22:E1554
Sadeghi Ekbatan S, Li XQ, Ghorbani M, Azadi B, Kubow S (2018) Chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-phase cell-cycle arrest and apoptosis in human colon cancer caco-2 cells. Int J Mol Sci 19:E723
Gokbulut AA, Apohan E, Baran Y (2013) Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology 18:144–150
Munshi A, Hobbs M, Meyn RE (2005) Clonogenic cell survival assay. Methods Mol Med 110:21–28
Kudugunti SK, Vad NM, Ekogbo E, Moridani MY (2011) Efficacy of caffeic acid phenethyl ester (CAPE) in skin B16-F0 melanoma tumor bearing C57BL/6 mice. Investig N Drugs 29:52–62
Pramanik KC, Kudugunti SK, Fofaria NM, Moridani MY, Srivastava SK (2013) Caffeic acid phenethyl ester suppresses melanoma tumor growth by inhibiting PI3K/AKT/XIAP pathway. Carcinogenesis 34:2061–2070
Funding
The authors would like to thank the financial support of CAPES [CAPES/PROEX—Process Numbers: 23038.005848/2018-31 and 88882.182142/2018-01] and CNPq [MDB Project. No. 449485/2014-5], Brazil. Funding sources are non-profit governmental agencies and had no role in study design, in the collection, analysis, and interpretation of the data, in the writing of the manuscript, and in the decision for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they no conflicts of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Infromed consent was obtained from all individual participants in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pelinson, L.P., Assmann, C.E., Palma, T.V. et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol Biol Rep 46, 2085–2092 (2019). https://doi.org/10.1007/s11033-019-04658-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04658-1