Abstract
Mourning constitutes an important human emotion, which might cause—among other things—major depressive symptoms when lasting for too long. To date, no study investigated whether mourning is related to specific psychophysiological activation patterns. Therefore, we examined physiological reactions induced by iconographic mourning-related stimuli in comparison to neutral and attachment stimuli in healthy adults (N = 77, mean age: 21.9). We evaluated pupillometric and eye-tracking parameters as well as heart rate variability (HRV) and skin conductance (EDA). Eye-tracking revealed a stronger dilated pupil during mourning in comparison to the neutral, but not to the attachment condition; furthermore, fixation patterns revealed less fixations on mourning stimuli. While HF HRV was reduced during mourning and attachment, we found no differences concerning EDA parameters between conditions. Results suggest specific eye-movement and pupil adaptations during representations of mourning, which might point toward inward cognition or avoidance, but no specific physiological pattern concerning HRV and EDA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mourning is generally understood as a natural reaction to the loss of loved ones or personally relevant objects. It can be caused by anything that leads to a permanent interruption of meaningful ties between a person and their environment (Papa & Maitoza, 2013) and might lead to major depression (Friedman, 2012). Furthermore, mourning has a specific signaling function. The prototypical expression of mourning might motivate others to provide help and support (Harrison et al., 2006; Miller et al., 2016). However, beyond the social-communicative component, emotional states also lead to cognitive adaptations (Mather et al., 2016; Schwabe, 2013, 2016; Schwabe & Wolf, 2013), changing how we cognitively represent and process information (Nesse & Ellsworth, 2009). Despite the importance of the function of mourning, the mourning response associated with it is unknown on a psychophysiological level. Current research mainly focuses on psychophysiological response patterns of basic emotions e.g., anger, fear, and happiness (e.g., Davydov et al., 2011; Overbeek et al., 2012; Prkachin et al., 1999). Investigating psychophysiological activity during the perception of mourning-relevant stimuli might yield important insights for potential treatment of prolonged mourning, as it marks one possible path to major depression and thus might accompany similar psychophysiological response patterns as well as symptoms (Friedman, 2012; Shear et al., 2013; Shear, 2015).
During mourning, the human attachment system is activated. From an evolutionary perspective, the attachment system can be regarded as a motivational system, which maximizes our chances for survival. In children, the attachment system is activated by attachment-related threats (e.g., separation from caregivers), or by inherently threatening situations (e.g., isolation, illness, injury). In adults, it is activated by attachment-related stress, such as the loss of a loved one, or through a broader set of emotional or interpersonal situations (Bowlby, 1969, 1980). Thus, in the present study, we not only applied neutral, but also attachment-related stimuli as a control condition to be able to investigate whether mourning-related scenes are distinct from attachment-related ones in terms of their psychophysiological response pattern. Those stimuli represent relationship scenes that intend to activate the attachment system of the viewer (Labek et al., 2016). By comparing mourning to attachment, we are able to differentiate whether the psychophysiological response is specific to the threat/stress component during mourning or related to attachment in general.
Experimental emotion research made valuable contributions to the clarification of the functional character of mourning. Emotional states, depending on their motivational intensity i.e., the extent to which they mobilize behavioral responses and thus increase behavioral vigor, either lead to a narrowing or widening of the spectrum of information that is cognitively represented and processed (e.g., Harmon-Jones et al., 2012, 2013; Maran et al., 2017; Mather & Sutherland, 2011; Mather et al., 2016). Experimental findings point to the close connection between emotion and motivation (Lang, 2010; Lang & Bradley, 2010, 2013; Maran et al., 2015) and locate emotions at specific points during goal pursuit (Nesse & Ellsworth, 2009). While pre-goal emotions constitute states of high motivational intensity encouraging the overcoming of obstacles in goal pursuit, post-goal emotions are located after goal failure and thus mark emotions of low motivational intensity (Gable & Harmon-Jones, 2010; Nesse & Ellsworth, 2009). Emotions of high motivational intensity narrow the cognitive focus on goal-relevant information, whereas emotions of low motivational intensity led to a broader focus (Gable & Harmon-Jones, 2010; Maran et al., 2015, 2017; Mather & Sutherland, 2011). Existing models describing emotion according to its motivational intensity and its consequences for cognitive functioning (e.g., Gable & Harmon-Jones, 2010; Lang, 2010; Lang & Bradley, 2010, 2013; Maran et al, 2015, 2017; Mather & Sutherland, 2011; Mather et al., 2016) classify mourning as an emotion of negative valence and low motivational intensity (Gable & Harmon-Jones, 2010). The state of mourning is thus located after goal failure, which also indicates inward thinking (Nesse & Ellsworth, 2009; Shear, 2015).
On first sight, mourning seems to be similar to the experience of sadness. However, these two emotions distinguish themselves in two key aspects: Firstly, while mourning is rated as highly arousing (e.g., Labek et al., 2017; Panayiotou, 2008), sadness is mostly related to low to moderate arousal (Brehm et al., 1999; Javela et al., 2008), when individuals rate mourning or sadness related stimuli subjectively. Secondly, while sadness resembles a more personal situation, mourning mostly describes situations that pertain to others. Although sadness is an emotion that might be caused by the loss of a loved person, it is defined in a broader sense than mourning. It thus might comprise various circumstances such as the failure to achieve a goal or losing sense of control (Ekman, 1999). On the other hand, the definition is stricter, as it is concerned with one specific emotion, whereas mourning might include different and more complex emotions, involving both positive and negative conditions (e.g., sadness, guilt, fear, gratitude, positive thoughts about beautiful experiences). Earlier studies supported the notion that emotional responses reflected basic dimensions of valence and arousal, but those scripts might be classified into specific emotion categories, indicating that a hybrid model may be most appropriate (Panayiotou, 2008). Mourning-associated images were derived from previous studies (Labek et al., 2016, 2017), which validated those images as highly arousing but low in valence in comparison to neutral ones. Furthermore, it should be noted that low motivational intensity is not the same as low arousal. Although mourning is an emotional state of low motivational intensity and thus might be accompanied by inaction just like major depression for example, it might be highly emotionally arousing at the same time.
Motivational intensity and arousal might be measured by means of psychophysiological activity (Lang & Bradley, 2010, 2013). During the perception of either pleasant or unpleasant arousing stimuli, an increased metabolic load as well as sensory adaptation have been shown (Bradley et al., 2008). Thus, our research question is concerned whether there are differences between mourning and control conditions (i.e., neutral and attachment) in terms of psychophysiological reactivity. Indeed, recent studies showed that sad stimuli trigger distinct psychophysiological patterns. Findings revealed an elevated heart rate (Kreibig et al., 2007; Prkachin et al., 1999; Shirai & Suzuki, 2017), higher electro-dermal activity (EDA; Davydov et al., 2011; Shirai & Suzuki, 2017) as well as lower heart rate variability (HRV; Overbeek et al., 2012; Shi et al., 2017) during the perception of sad stimuli in comparison to happy and other. In general, sadness is characterized by a reduction of cardiovascular parasympathetic control (e.g., Brunoni et al., 2013; Hoffmann et al., 2017) and an increase of sympathetic control (Kreibig, 2010). Therefore, based on earlier results and as sadness and mourning share quite an amount of overlap with both emotional states potentially leading to major depression, we would expect similar psychophysiological patterns induced by mourning-related content concerning EDA and HRV. Furthermore, both sadness and mourning include mainly negative valence as well as high arousal on the physiological level (Labek et al., 2017; Shirai & Suzuki, 2017). In the present study, we decided to evaluate high frequency HRV (HF HRV) as an important index of parasympathetic activity (Berntson et al., 2007; Thayer & Lane, 2007). Theoretical frameworks like the polyvagal theory (Porges, 2007) and the neurovisceral model (Thayer & Lane, 2009) suggested a close connection between HRV and emotion regulation. Moreover, lower levels of HRV are associated with negative emotional states (Appelhans & Luecken, 2006), while higher HRV is suggested to be protective for depressive symptoms. Moreover, certain brain networks e.g., the ventromedial prefrontal cortex, insula, amygdala, and cingulate are both involved in emotional processing and the autonomic control of the heart rate (Thayer et al., 2012). Moreover, earlier studies found HRV to be an adequate tool for evaluating human emotions in highly arousing visual stimuli (Choi et al., 2017). EDA, on the other hand, constitute measures of the sympathetic nervous system and represents changes in the electrical activity of palmar and plantar skin, the skin conductance (Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures, 2012). EDA is commonly applied in dyadic interaction studies (e.g. Duijndam et al., 2020) and for measuring emotional arousal in response to sad or emotionally distressing films (e.g. Dunn et al., 2009; Kreibig et al., 2007). Dawson et al. (2000) claimed that EDA is not a clearly interpretable measure of any psychological process in particular, but in a well-controlled experimental paradigm, a conclusive interpretation of a psychological meaning of an electro-dermal response becomes interpretable when looking at specific stimulus conditions as we did in our experiment. Both HF HRV and EDA measures then enable an integrative assessment of the different influences of sympathetic and parasympathetic branches of the autonomic nervous system, as they are considered relatively pure indices of those nervous system branches. They further allow us to quantify emotion regulatory capacities (Berntson et al., 2008) and give an insight into the dynamic changes in the states of emotional arousal that occur during sad picture viewing.
In addition, we are going to evaluate pupil dilation during the processing of mourning stimuli, which is another important indicator for the activation of the sympathetic system (Bradley et al., 2008; Sirois & Brisson, 2014). To date no study has attempted to do so. Both changes in pupil dilation and EDA are indicative of emotional arousal (Granholm & Steinhauer, 2004; Bradley et al., 2008). They thus constitute a valuable and reliable measure of an individual’s response to emotional stimuli (Sirois & Brisson, 2014). Moreover, recent findings suggest that pupil dilation and its tonic component are sensitive to mind wandering. Thus, inward cognition might be detected by changes in pupil width (Franklin et al., 2013; Grandchamp et al., 2014). Eye-tracking parameters not only provide insight into the social (Hoffmann et al., 2020; Maran et al., 2020) and personality factors underlying gaze behavior (Maran et al., 2019), but also further information about the directionality of an individual’s current cognitive state i.e., whether it is directed internally or externally (Benedek et al., 2017). Internally directed cognition is often accompanied by gaze aversion, which further enhances visual imagination (Buchanan et al., 2014; Vredeveldt et al., 2011). Insight solutions are preceded e.g., by gazing away from the stimulus, which might be viewed as a disconnection from visual input during periods of insight (Salvi & Bowden, 2016; Salvi et al., 2015).
These findings point towards eye-movement actively facilitating internally directed cognition by reducing visual information processing to avoid disruption through sensory stimulation. Eye-movement patterns indicating inward cognition are also associated with emotional processing (Dixon et al., 2014). Internally directed cognition describes active cognitive processes that are not oriented towards external stimuli and are closely related to memory processing (Andrews-Hanna et al., 2014). Furthermore, these findings are consistent with findings on eye-movement patterns in mind wandering (Reichle et al., 2010; Smilek et al., 2010; Uzzaman & Joordens, 2011). Pupillometry and eye-tracking constitute sensitive measuring instruments for mourning relevant stimuli (Harrison et al., 2006; Siegle, et al., 2003). In fact, pupillary dynamics during the viewing of mourning stimuli are a predictive marker for the development of mourning-like states (Burkhouse et al., 2015).
Due to the above-mentioned findings, we argue that (1) during mourning stimulation, eye-movement patterns, which indicate internally directed cognition would occur more frequently (Benedek et al., 2017). Since mourning as a post-goal emotion is associated with low motivational intensity (Gable & Harmon-Jones, 2010; Nesse & Ellsworth, 2009), it should lead to an expansion in cognitive focus and thus induce more inwardly directed cognitive exploration e.g., less, and shorter fixations on mourning related stimuli. Thus, we would await, the duration of the first fixation as well as the total fixation duration to be shorter in comparison to neutral and attachment stimuli; furthermore, the time to first fixation would be slower, while the number of fixations on mourning stimuli should be lower than in neutral and attachment. All these eye-tracking indices would prove an avoidant gaze-behavior during the induction of mourning. (2) On the other hand, increased internally directed cognition during mourning should lead to a similar pattern of pupil dynamics like current findings for mind wandering i.e., a stronger dilated pupil during mourning than during neutral and attachment stimuli (Reichle et al., 2010; Uzzaman & Joordens, 2011). A stronger dilated pupil would also be in line with earlier research showing mourning to be a highly arousing emotional state (e.g., Labek et al., 2017). (3) As other indicators for the sympathetic nervous system, we further assume EDA to be enhanced during presentation of mourning related stimuli compared to neutral and attachment stimuli. There are contrasting results from earlier studies showing avoidance-related sadness leading to enhanced EDA, whereas attachment-related sadness led to reduced EDA (Davydov et al., 2011; Shirai & Suzuki, 2017). From our line of argument, mourning is more closely related to avoidance in accordance with the assumption of gaze behavior to be avoidant, thus leading to enhanced EDA. Last, (4) HRV will be reduced during the viewing of mourning stimuli compared to neutral and attachment. This assumption is based on earlier studies showing reduced HRV during sadness induction (Overbeek et al., 2012) as well as a reduction of cardiovascular parasympathetic control in depressed individuals (e.g., Brunoni et al., 2013; Hoffmann et al., 2017).
Methods
Participants
Our study sample consisted of 77 adult volunteers (51 female, 26 male); their mean age was 21.9 years (range 18 to 44; SD = 3.56). Due to data loss during physiological recording (i.e., detached electrodes), only 43 subjects were included in the reported analysis of EDA data (20 male, 23 female; mean age: 21.9). No data was lost during eye-tracking or ECG recordings for any of our participants. Each of the participants had normal or corrected to normal vision. Subjects with glasses were asked to wear contact lenses instead. Thus, exclusion criteria were a corrected vision by glasses (no eye-tracking possible with glasses) as well as any kind of physiological or psychological condition. All subjects were Caucasians, mainly students from the first author’s University. The study was conducted in line with the guidelines of the Declaration of Helsinki and informed consent was provided.
Stimuli
Stimuli constitute iconographic representations of scenes that represent neutral interactions, interactions relevant to mourning, and ambiguous relationships. Ambiguous scenes were taken from the Adult Attachment Projective Picture System (AAP; George & West, 2001). The AAP allows capturing attachment styles through iconographic visual stimuli. The stimuli represent attachment relationships or potential relationships that intend to activate the attachment system of the viewer (Labek et al., 2016). The neutral and mourning-associated images were derived from previous studies (Labek et al., 2016, 2017), which also validated the mourning images as highly arousing and low in valence in comparison to neutral ones (see Supplementary Material of Labek et al., 2017). The strong association of certain body configurations with the depiction of mourning persons i.e., culturally mediated postures in dyads, enables the creation of prototypical drawings that induce mourning or cognitions associated with mourning (see Fig. 1). In our study, the representations of attachment as well as mourning were examined in iconographic forms (Labek et al., 2017). Visual stimuli had the same RMS-contrast and luminance across all three conditions; all pictures were black and white.
Example pictures from the mourning (first row), attachment (second row), and neutral stimuli (third row; reproduced with consent from Labek et al., 2017)
We further evaluated the visual complexity of each picture by counting the number of figures (open or closed) as well as the number of vertical, horizontal, and diagonal lines. This is a valid method to quantify visual complexity, especially in simple drawings (Pieters et al., 2010). Moreover, we applied the canny edge detection algorithm (Canny, 1986), which is a widely used tool to detect weak edges appearing in combination with strong edges in grayscale images. The sensitivity threshold was automatically determined. We used JPEG compression files from the images for the application of the Canny-algorithm, and determined a raw measure of the number of black pixels. We then ran one-way ANOVAs to test whether the pictures of each condition differed significantly in any of these complexity measures and found no significant differences between conditions (all p’s > 0.05; see Table 1). As can be seen in Fig. 1, gravestones as well as body postures (e.g., bowed head, hands to face) associated with mourning were presented in the mourning stimuli; these can be easily identified as such (Labek et al., 2017). The authors have not considered crying facial expressions or tears, since their intent was to differentiate the mourning stimulus set from those showing emotional facial expressions. For attachment, depicted scenes show relationship scenes e.g., a couple or a mother and their child.
Research design
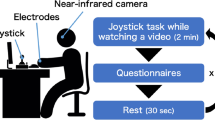
First, subjects were provided with information about the course of the study and informed consent. The Ethics Advisory Board of the local University classified the entire course of the experiment as harmless. At the beginning and at the end of the experiment, subjects watched a relaxing film (Piferi et al., 2000; Pijper, et al., 2018) followed by a fixation cross for measuring baseline pupil diameter, EDA, and HRV. Subsequently, subjects were presented with picture sets in three pseudo-randomized runs, containing eight pictures each. Pictures were presented 12 s each with an inter-stimulus interval of 10 s (22 s per trial, total duration: ~ 10 min). The presentation order of the blocks including mourning- and attachment-relevant interactions was alternated. Images with neutral content were always presented in the middle. After each stimulus set there was a baseline condition with a fixation cross (see Fig. 2). Subjects were asked to sit straight in front of the monitor without crossing arms or legs. After a 9-point calibration of the eye-tracker, subjects were verbally instructed to relax, ideally not to move and to just look at the pictures. Participants were instructed before the task: “Now you are going to see a set of pictures. Just have a look at them; you do not have to act.” In addition to the eye-tracking task, subjects were asked about their current mood before and after the testing session as well as after each of the three condition blocks to check if certain stimuli would have an impact on our subjects’ mood (How do you feel now? How happy/angry/sad/anxious/surprised are you now?).
Mood manipulation check
To examine whether the pictures within each condition influenced our subjects’ mood, we calculated paired samples t-tests (one-tailed), and thus compared the mood values before and after each condition with one another. We found a significant difference between baseline and mourning condition (t(77) = − 1.65, p = 0.05). The difference between baseline and neutral (t(77) = 0.82, p = 0.21) as well as attachment (t(77) = − 0.71, p = 0.24) was not significant.
Eye-tracking
The eye-tracking task was presented on a standard desktop computer (distance to screen: 60 cm; resolution: 1920 × 1080; refresh rate: 60 Hz; Dell Precision T-5610). We applied a Tobii TX-300 (gaze-sampling frequency = 300 Hz, gaze sampling variability < 0.3%; accuracy = 0.4°, precision = 0.14°) for video eye-tracking, which took place in a normally lit room with blinds blocking sunlight from outside; thus, lighting conditions were consistent for all participants. We exported the following parameters: time to first fixation, first fixation duration, total fixation duration, and fixation count within a predefined area of interest (AOI) that included the whole of each stimulus picture, i.e. the entire square via Tobii Pro Studio software. The picture stimuli did not fill the whole of the screen but were presented in the center (size 900 × 755px). The background was white, framing the picture from four sides. Therefore, avoidant gaze behavior outside the box was also a possibility, which indicated no fixations on the stimulus. Furthermore, there is always the possibility that subjects close their eyes during stimulus presentation or look away from the screen when they do not enjoy looking at the presented stimulus material. Thus, we argue that these parameters are indeed of great relevance for assessing participants’ reactions to the stimuli.
The time to first fixation is the time it takes before the AOI is fixated for the first time following the stimulus onset. The first fixation duration is the length of this first fixation. Fixation count and total fixation duration are defined as the number and length of all fixations within the AOI over the whole course of the stimulus presentation. Tobii Pro Studio further offers to export the so-called visit parameters, which are basically parameters, where no fixations on concrete points are recorded, but the time or frequency in which gaze points are recorded somewhere in the AOI. Accordingly, we chose the more conservative parameters, as with visits there is a risk that random gazes and non-directive gazes are also captured in the metric. We computed average scores across all eight stimuli within each condition and z-transformed all scores before further analysis.
Pupil diameter measurements were processed using the open-source tool Cheetah Experimental Platform Web 2.0 (CEP–Web; Zugal et al., 2017). First, any missing values from one pupil were substituted by the values of the respective other pupil. Second, we removed all values that differed more than three standard deviations from the mean, as they were considered outliers. Third, the implemented blink detection filter based on a heuristic of missing values and gaze position, detects, and clips out blinks 200 ms section before and after each identified blink (Pedrotti et al., 2011). Next, missing data was linearly interpolated by values just before and after each blink. Finally, based on the continuous pupil measurements, data were low pass filtered using a third order low pass Butterworth filter with a cutoff frequency of 4 Hz. We removed measurements within the first 2000 ms since they reflected the initial pupillary adaptation to the low-level differences between the pictures and the following fixation cross. To calculate relative pupil values, we divided values from relevant time spans by their baseline before stimulus-onset i.e., the last 2000 ms from the fixation cross before.
Thus, for all images, we calculated a mean value over the entire stimulus duration relative to the baseline (tonic pupil dilation). We therefore calculated the baseline-corrected tonic pupil dilation across the whole course of stimulus presentation (average score across all eight pictures of each set), where we would be able to see whether changes in pupil dilation hold true over the whole course of stimulus presentation or not.
Physiological recordings
We obtained an ECG at a sampling rate of 500 Hz by placing three electrodes at the mid-clavicle, solar plexus and lowest right rib using a varioport-e data recorder and the biometrics software suite (BiSigma, Freiburg, Germany). ECG recordings were offline filtered using a high pass filter (cut-off frequency 3 Hz). R-R intervals were extracted from ECG by an R-peak detection algorithm using Kubios software (Tarvainen et al., 2009). For artifact correction, we applied the built-in automatic artifact correction algorithm from Kubios software, which applies a time varying threshold to separate ectopic and normal beats within successive R-R intervals. We further screened for artifacts visually and removed any misplaced or wrongly detected beats. Moreover, a 3 Hz cubic spline interpolation was applied to form equidistantly sampled time series from the IBI data. We then computed HRV parameters. Frequency domain analyses were applied, separating individual R-R intervals into frequency bands, and determining the power of each band. Fast Fourier transformation was further used to transform R-R intervals into high-frequency bands (HF HRV). Thus, HF HRV was indexed by spectral power density in the frequency range 0.15–0.40 Hz (Berntson et al., 1997). We selected the smoothing option of the software to remove possible low trends in the data. For calculating HF HRV values (absolute power, ms2) for the three different conditions (neutral, attachment, and mourning), we computed HRV parameters across all stimuli within each condition (epoch length per condition: 176 s). Due to violation of the normality assumption, HRV scores were log-transformed (natural logarithm) prior to statistical analysis.
To obtain EDA data, we placed two electrodes on the thenar and hypothenar of participants’ non-dominant hand; we recorded EDA data at a frequency of 500 Hz using the varioport-e data recorder as well as the biometrics software suite (BiSigma, Freiburg, Germany). Before the recording, we instructed participants to wash their hands without soap to reduce skin impedance. For analysis, the data were down sampled to 125 Hz. The phasic and tonic component of EDA are expressed in microsiemens (μS) and computed with Ledalab V3.4.9 software. Artifacts were corrected with the built-in artifact correction in the preprocessing of Ledalab software. We further visually screened for artifacts. We chose the continuous decomposition analysis, which is the extraction of continuous phasic/tonic activity based on standard deconvolution (Benedek & Kaernbach, 2010). Parameters, which can be analyzed with Ledalab software, are: (1) The number of significant skin conductance responses (SCR), (2) The response latency of the first significant SCR, (3) The sum of SCR-amplitudes of significant SCRs, (4) The average phasic driver within the response window, (5) Area (i.e. time integral) of phasic driver, (6) The maximum value of phasic activity, and (7) The mean tonic activity (of decomposed tonic component).
Tonic and phasic EDA values as well as peak amplitudes are common metrics to be evaluated (Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures, 2012). For peak amplitudes of the psychophysiological responses, a minimum amplitude to be counted as a response was defined at greater than 0.03 μS, as it occurred within 1 to 4 s after stimulus onset. The tonic component was computed as the mean change during the period of stimulus presentation (12 s), which means that phasic components were subtracted from the mean level. Phasic EDA represents phasic activity within the response time window but does not fall back on classic SCR amplitudes. EDA scores were z-transformed prior to statistical analysis. We used Kendall H135 electrodes (Ø 35 mm) for the ECG recording and Kendall H124 electrodes (Ø 24 mm) for the EDA recording, where an Ag/AgCl sensor was embedded in an adhesive as well as conductive liquid hydrogel. The carrier material was foam (adhesive).
Statistical analysis
For the statistical analysis of eye-tracking indices (time to first fixation, first fixation duration, total fixation duration, fixation count), pupil width, tonic, phasic, and peak EDA, as well as LF and HF HRV, we computed Linear Mixed Effects models with a random intercept for our subjects and with experimental condition (neutral vs. mourning vs. attachment) as the predictor. We further correlated pupil width and eye-tracking indices to explore condition specific variations. In case of a significant main effect of condition, we report pairwise comparisons, which we adjusted for multiple comparisons (Bonferroni correction). Alpha levels were set at 0.05. Results are reported with original df, corrected p-values as well as 95% confidence intervals. Data were analyzed using SPSS 25.0.
Sensitivity power analysis with G*Power (Faul et al., 2009) showed that a sample size of N = 43 was sufficient to detect a medium-sized effect of f = 0.5 with a statistical power of 1 − β = 0.95 and α = 0.05 in a within subjects repeated measures ANOVA model. This sample size should be sufficient for the random intercept model of the present study since the latter has generally more power (Harrison et al., 2018). Effect sizes for EDA measures during sad picture viewing are ranging between \({\eta }_{p}^{2}\) = 0.31 to 0.35 (Davydov et al., 2011; Shirai & Suzuki, 2017), while for HRV \({\eta }_{p}^{2}\) = 0.20 to 0.31 were found (Overbeek et al., 2012). Sample size was determined before any data analysis.
Results
Fixation behavior
While the time to first fixation did not differ between conditions (F[2,227] = 1.02, p = 0.362, see Fig. 3A), the duration of the first fixation differed depending on experimental condition (F[2,227] = 25.56, p < 0.001; see Fig. 3B). Neutral stimuli were focused shorter than attachment (MD = − 0.24, p < 0.001, CI2.5% = − 0.35, CI97.5% = − 0.13) and mourning stimuli (MD = − 0.30, p < 0.001, CI2.5% = − 0.41, CI97.5% = − 0.20). The latter did not differ significantly from each other, though (MD = − 0.07, p = 0.411, CI2.5% = − 0.04, CI97.5% = 0.18).
The total fixation duration also differed between conditions (F[2,227] = 3.35, p = 0.037). Attachment stimuli were focused longer than neutral ones (MD = − 0.18, p = 0.031, CI2.5% = − 0.34, CI97.5% = − 0.01). We found no significant differences between the other conditions. For the total number of fixations on stimulus images, a significant main effect for condition was found (F[2,227] = 38.31, p < 0.001). Both attachment (MD = − 0.29, p < 0.001, CI2.5% = − 0.41, CI97.5% = − 0.16) and mourning (MD = 0.17, p = 0.004, CI2.5% = 0.04, CI97.5% = 0.30) differed significantly from the neutral condition; attachment stimuli received significantly more fixations than mourning (MD = 0.46, p < 0.001, CI2.5% = 0.33, CI97.5% = 0.58).
Pupil dilation
For the tonic pupil response, there was also a difference across conditions (F[2,228] = 3.85, p = 0.023; see Fig. 4). While the difference between neutral and mourning was meaningful (MD = 0.02, p = 0.034, CI2.5% = 0.00, CI97.5% = 0.03), this was not true for the difference between neutral and attachment (MD = 0.01, p = 0.081, CI2.5% = 0.00, CI97.5% = 0.03). Reactions were similar for both attachment and mourning representations (MD = 0.00, p = 1.000. CI2.5% = − 0.01, CI97.5% = 0.02), though. Additionally, correlation analysis revealed a negative relation between the tonic pupil response and the total fixation duration (r(75) = − 0.36, p = 0.001) as well as the time to first fixation (r(75) = − 0.21, p = 0.035) in mourning stimuli only.
Physiological indices
For HF HRV, we found a significant main effect for condition (F[2,228] = 23.63, p < 0.001; see Fig. 5). Pairwise comparisons showed a significant difference between neutral and attachment (MD = 0.78, p < 0.001, CI2.5% = 0.48, CI97.5% = 1.08) as well as mourning stimuli (MD = 0.71, p < 0.001, CI2.5% = 0.41, CI97.5% = 1.01), but not between attachment and mourning (MD = 0.07, p = 1.000, CI2.5% = − 0.23, CI97.5% = 0.37). For EDA, neither the phasic (F[2,122] = 2.66, p = 0.074) nor the tonic parameter (F[2,80] = 0.32, p = 0.729) differed between conditions. Moreover, peak amplitudes did not differ between conditions (F[2,169] = 2.61, p = 0.077).
Discussion
The present study investigated psychophysiological patterns in response to mourning stimuli in a sample of healthy young adults. Pupil dynamics and eye-tracking parameters have never been investigated in response to mourning stimuli before. Moreover, the mourning response was unknown on a psychophysiological level, as current research mainly focused on the psychophysiological responses induced by basic emotions, for example anger, happiness, or sadness. Moreover, in contrast to other studies investigating psychophysiological activation patterns, stimuli represented iconographic scenes, which allow for interpretations that are more subjective.
In support of our first hypothesis, we found specific eye-movement patterns in response to mourning compared to neutral and attachment stimuli, which point towards an expansion in cognitive focus and thus more inwardly directed cognitive exploration. This result stands in line with the notion that mourning is a post-goal emotion associated with low motivational intensity (Gable & Harmon-Jones, 2010; Nesse & Ellsworth, 2009). In addition, pupil dynamics revealed increased pupil excitation in response to mourning stimuli compared to neutral ones, which stands in line with the notion that mourning is highly arousing (Labek et al., 2017). The difference between mourning and attachment scenes was not meaningful, thus indicating that both kinds of stimuli produced similar arousal levels. Similar as in studies investigating mind wandering, pupils dilated more when being off task or off stimulus, as shown by a significant negative correlation between the tonic pupil response and the time to first fixation as well as total fixation duration in mourning stimuli (Franklin et al., 2013). As such, our results confirm that inward cognition and mind wandering lead to an increase in pupil dilation. Another explanation for this finding might be an avoidant gaze behavior, which we also hypothesized.
In addition to pupil dilation, we further evaluated EDA (Berntson et al., 2007), which is another indicator for the sympathetic nervous system. None of the evaluated EDA parameters differed significantly between conditions, which makes the result of pupil dilation more difficult to interpret. Just as we hypothesized, HF HRV was lower during mourning compared to neutral stimuli. Nevertheless, this pattern was the same for attachment stimuli. Thus, our results only partially support our third hypothesis by showing reduced HRV as a response to mourning stimuli, as they do not seem to be specific to mourning.
As stated in the theoretical background, pupillometry and eye-tracking are reliable methods, which allow for tracking active cognition (Benedek et al., 2017). Especially pupil dynamics and fixation parameters can explain underlying cognitive and motivational as well as arousal mechanisms during emotional processing (Lang & Bradley, 2010). As mourning is classified as an emotion of negative valence and low motivational intensity (Gable & Harmon-Jones, 2010), and thus should lead to more inward cognition, we hypothesized less and shorter fixations in mourning compared to neutral and attachment stimuli. Furthermore, pupils should be dilated more during mourning than during neutral and attachment stimuli, as they are also highly arousing (Labek et al., 2017). The former expectation could partially be confirmed as mourning pictures were indeed fixated less often than neutral and attachment ones. In terms of fixation duration, no significant differences between attachment and mourning could be obtained, although both conditions differed significantly from the neutral one; contrary to our hypothesis, mourning stimuli were fixated longer than neutral ones.
Our hypothesis of HF HRV as an index of parasympathetic cardiac control being lowered during mourning induction as compared to neutral and attachment received only partial support. This hypothesis was based on earlier results of psychophysiological activity during the induction of sadness (Overbeek et al., 2012) as well as other studies showing reduced HF HRV in sad and depressed individuals (Brunoni et al., 2013; Hoffmann et al., 2017). According to its motivational intensity, mourning is classified as an emotion of low motivation. Thus, we would have awaited HF HRV to be lowered in response to mourning stimuli. As hypothesized, HF HRV was lower in response to mourning as to neutral stimuli. Although the difference between the neutral and mourning condition was significant, we could not find any difference between attachment and mourning. This speaks for no special HF HRV pattern in mourning compared to attachment stimuli, but for a general low motivational intensity during mourning and attachment related scenes.
In addition to pupil dilation, we further evaluated EDA parameters, which are important markers for sympathetic nervous system activity (Berntson et al., 2007). In contrast to our hypotheses and results from earlier studies showing specific physiological patterns during sadness (Davydov et al., 2011; Shirai & Suzuki, 2017), we found no meaningful differences between conditions for any these parameters. Sadness related to avoidance led to enhanced EDA whereas sadness related to attachment led to reduced EDA compared to baseline values (Shirai & Suzuki, 2017). A possible explanation for these latter findings might be that, as stimuli in the study represented iconographic scenes, which allow for subjective interpretations, mourning induction was insufficient. As conditions did not differ from one another significantly, one might argue that there was no special psychophysiological pattern during the induction of mourning. This result stands in contrast to earlier studies showing elevated heart rate, blood pressure and stroke volume (Kreibig et al., 2007; Prkachin et al., 1999; Shirai & Suzuki, 2017) as well as HRV (Overbeek et al., 2012) during sadness induction. Our results might be due to the experimental setting where we confronted our subjects with the stimuli on a viewing-basis only without any additional instruction. Therefore, arousal levels might not have been high enough to produce cardiac sympathetic changes in addition to changes in pupil dilation.
Other studies used different kinds of stimuli to induce emotions e.g., emotion imagination, color pictures depicting real-life scenes and/or film clips. More complex stimuli e.g., film clips were found to elicit a more accurate emotional experience and specific arousal pattern than components presented separately (Baumgartner et al., 2006). Moreover, any method to induce mourning might be superficial compared to a real-life experience of mourning, as it has indeed many facets, which simple picture stimuli might not be able to cover e.g., about who or what does someone mourn, the kind of relationship that lies behind the mourning, the kind of mourning itself (i.e., acute, prolonged, complicated). Thus, mourning constitutes a more complex emotion, which might not as easily be induced as sadness, happiness, or fear for example. Projective stimuli like those used in the present investigation might not be eliciting any specific emotional arousal, as mourning stimuli did not differ from attachment stimuli in physiological parameters i.e., EDA and HRV. Nevertheless, we found specific pupillary variations and eye-movement patterns, which speak for a special processing of mourning related stimuli. Finally, yet importantly, mourning might be too closely related to attachment, as attachment is also defined as a sub-facet of mourning. Just like sadness might be related either to avoidance or attachment (Shirai & Suzuki, 2017), the same counts for mourning. Like mentioned in the introduction of this paper, during mourning, the human attachment system is activated for example by the loss of a loved one, or through a broader set of emotional or interpersonal situations (Bowlby, 1969, 1980). Thus, with the results of the present study, we argue that the psychophysiological response pattern of mourning-related scenes is not distinct from attachment-related ones. For our understanding of mourning as an emotion, this highlights the notion that it is deeply connected with attachment.
Limitations
First, our sample consists of predominantly young adults. Given their developmental stage, they are less likely to have mourned the passing of an immediate family member other than grandparents, compared to older adults. Thus, it would be helpful to investigate the psychophysiological response to mourning stimuli in older adults in future research. Developmental differences in responses to mourning stimuli across the lifespan could be investigated. In an additional analysis, we split our sample into two sub-groups—one group, who experienced the loss of a close friend/relative (N = 47) and one group, who experienced a break-up only (N = 30). The results can be found in Table 2 and indicate no significant group by condition interactions for any of the variables of interest apart from the first fixation duration being longer in the death than in the break-up group for mourning stimuli only. Unfortunately, we did not check how self-relevant the participants experienced the mourning scenes. This would have been helpful for evaluating whether the shown scenes pertained to a sense of personal loss or just sadness in general. Second, we did not compare our mourning stimuli to sadness or other relevant conditions e.g., happiness. Stimuli including gravestones might be easily associated with mourning, but other stimuli showing individuals with a bowed head or hands to face might not only be associated with mourning, but with feeling sad or overwhelmed in general. Thus, it is difficult to propose a physiological response specific to mourning. Nevertheless, those stimuli were specifically developed and validated to induce a sense of mourning (Labek et al., 2016, 2017).
Future research should examine the difference between iconographic and other kinds of stimuli. Earlier studies applied techniques like individual emotion description (Prkachin et al., 1999), film clips (Davydov et al., 2011; Kreibig et al., 2007; Overbeek, et al., 2012), mental imagery (Shirai & Suzuki, 2017), and color pictures depicting real-life scenes (Overbeek et al., 2012). We however were one of the first to employ projective stimuli in this kind of study context. Moreover, the pairing of physiological methods alongside fMRI (Labek et al., 2017) would be worthwhile to apply. We only used two control conditions i.e., neutral and attachment. Other control conditions e.g., happiness and sadness should be applied. Comparing the response to discrete emotional stimuli from individuals, who are mourning to those, who are not mourning could be another interesting target to examine. Choi et al. (2017) recommended applying an HRV-based evaluation only, when a high emotional level is achieved through visual stimulation. As we did not measure the emotional level, it is not clear if the emotional level was high enough to apply HRV- or EDA-based measures for example. Nevertheless, earlier studies using iconographic stimuli showed specific activation patterns, which differed from emotionally negative and high-arousing stimuli (Labek et al., 2017). Regarding HRV and EDA recordings, the constraint to one time of measurement might be insufficient as the temporal stability of HRV measures is limited. An estimation showed that in individual measurements, nearly 40% of the variance in HRV parameters is explained by situational and person-situation interaction effects (Bertsch et al., 2012); therefore, aggregation of HRV data across various measurements would improve reliability.
This study is the first to show specific eye-movement and pupillary patterns during the perception of projective mourning stimuli in comparison to neutral and attachment conditions. While we replicated earlier findings of decreased HRV parameters (Overbeek et al., 2012), we were not able to prove this as a specific pattern for mourning stimuli only. Nevertheless, our results may generate further insight into emotion regulation and motivational intensity theory (Lang & Bradley, 2010, 2013) and highlight the fact that motivational intensity and arousal constitute two sides of the story here.
Data availability
Materials and data associated with this research are available at Open Science Framework (https://osf.io/ardc8/).
Change history
02 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11031-022-09933-6
10 May 2022
A Correction to this paper has been published: https://doi.org/10.1007/s11031-022-09945-2
References
Andrews-Hanna, J. R., Saxe, R., & Yarkoni, T. (2014). Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage, 91, 324–335. https://doi.org/10.1016/j.neuroimage.2014.01.032
Appelhans, B. M., & Luecken, L. J. (2006). Heart rate variability as an index of regulated emotional responding. Review of General Psychology, 10(3), 229–240. https://doi.org/10.1037/1089-2680.10.3.229
Baumgartner, T., Esslen, M., & Jäncke, L. (2006). From emotion perception to emotion experience: Emotions evoked by pictures and classical music. International Journal of Psychophysiology, 60(1), 34–43. https://doi.org/10.1016/j.ijpsycho.2005.04.007
Benedek, M., & Kaernbach, C. (2010). A continuous measure of phasic electrodermal activity. Journal of Neuroscience Methods, 190(1), 80–91. https://doi.org/10.1016/j.jneumeth.2010.04.028
Benedek, M., Stoiser, R., Walcher, S., & Körner, C. (2017). Eye behavior associated with internally versus externally directed cognition. Frontiers in Psychology, 8, 1092. https://doi.org/10.3389/fpsyg.2017.01092
Berntson, G. G., Thomas Bigger Jr, J., Eckberg, D. L., Grossman, P., Kaufmann, P. G., Malik, M., Nagaraja, H. N., Porges, S. W., Saul, J. P., Stone, P. H., & Van Der Molen, M. W. (1997). Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology, 34(6), 623–648. https://doi.org/10.1111/j.1469-8986.1997.tb02140.x
Berntson, G. G., Quigley, K. S., & Lozano, D. (2007). Cardiovascular psychophysiology. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), Handbook of psychophysiology (pp. 182–210). Cambridge University Press.
Berntson, G. G., Norman, G. J., Hawkley, L. C., & Cacioppo, J. T. (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45, 643–652. https://doi.org/10.1111/j.1469-8986.2008.00652.x
Bertsch, K., Hagemann, D., Naumann, E., Schächinger, H., & Schulz, A. (2012). Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology, 49(5), 672–682. https://doi.org/10.1111/j.1469-8986.2011.01341.x
Bowlby, J. (1969). Attachment. Basic Books.
Bowlby, J. (1980). Loss. Basic Books.
Bradley, M. M., Miccoli, L., Escrig, M. A., & Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–607. https://doi.org/10.1111/j.1469-8986.2008.00654.x
Brehm, J. W., Brummett, B. H., & Harvey, L. (1999). Paradoxical sadness. Motivation and Emotion, 23(1), 31–44. https://doi.org/10.1023/A:1021379317763
Brunoni, A. R., Kemp, A. H., Dantas, E. M., Goulart, A. C., Nunes, M. A., Boggio, P. S., Mill, J. G., Lotufo, P. A., Fregni, F., & Benseñor, I. M. (2013). Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. International Journal of Neuropsychopharmacology, 16(9), 1937–1949. https://doi.org/10.1017/S1461145713000497
Buchanan, H., Markson, L., Bertrand, E., Greaves, S., Parmar, R., & Paterson, K. B. (2014). Effects of social gaze on visual-spatial imagination. Frontiers in Psychology, 5, 671. https://doi.org/10.3389/fpsyg.2014.00671
Burkhouse, K. L., Siegle, G. J., Woody, M. L., Kudinova, A. Y., & Gibb, B. E. (2015). Pupillary reactivity to sad stimuli as a biomarker of depression risk: Evidence from a prospective study of children. Journal of Abnormal Psychology, 124(3), 498–506. https://doi.org/10.1037/abn0000072
Canny, J. (1986). A computational approach to edge detection. IEEE Transactions on Pattern Analysis and Machine Intelligence, 6, 679–698. https://doi.org/10.1109/TPAMI.1986.4767851
Choi, K. H., Kim, J., Kwon, O. S., Kim, M. J., Ryu, Y. H., & Park, J. E. (2017). Is heart rate variability (HRV) an adequate tool for evaluating human emotions?–A focus on the use of the International Affective Picture System (IAPS). Psychiatry Research, 251, 192–196. https://doi.org/10.1016/j.psychres.2017.02.025
Davydov, D. M., Zech, E., & Luminet, O. (2011). Affective context of sadness and physiological response patterns. Journal of Psychophysiology, 25(2), 67–80. https://doi.org/10.1027/0269-8803/a000031
Dawson, M. E., Schell, A. M., & Filion, D. L. (2000). The electrodermal system. In J. T. Cacioppo, L. G. Tassinary, & G. G. Berntson (Eds.), The handbook of psychophysiology (2nd ed., pp. 217–243). Cambridge University Press.
Dixon, M. L., Fox, K. C., & Christoff, K. (2014). A framework for understanding the relationship between externally and internally directed cognition. Neuropsychologia, 62, 321–330. https://doi.org/10.1016/j.neuropsychologia.2014.05.024
Duijndam, S., Karreman, A., Denollet, J., & Kupper, N. (2020). Emotion regulation in social interaction: Physiological and emotional responses associated with social inhibition. International Journal of Psychophysiology, 158, 62–72. https://doi.org/10.1016/j.ijpsycho.2020.09.013
Dunn, B. D., Billotti, D., Murphy, V., & Dalgleish, T. (2009). The consequences of effortful emotion regulation when processing distressing material: A comparison of suppression and acceptance. Behaviour Research and Therapy, 47(9), 761–773. https://doi.org/10.1016/j.brat.2009.05.007
Ekman, P. (1999). Basic emotions. In T. Dalgleish & M. J. Power (Eds.), Handbook of cognition and emotion (pp. 45–60). Wiley.
Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149
Franklin, M. S., Broadway, J. M., Mrazek, M. D., Smallwood, J., & Schooler, J. W. (2013). Window to the wandering mind: Pupillometry of spontaneous thought while reading. Quarterly Journal of Experimental Psychology, 66(12), 2289–2294. https://doi.org/10.1080/17470218.2013.858170
Friedman, R. A. (2012). Mourning, depression, and the DSM-5. The New England Journal of Medicine, 366(20), 1855–1857. https://doi.org/10.1056/NEJMp1201794
Gable, P., & Harmon-Jones, E. (2010). The blues broaden, but the nasty narrows: Attentional consequences of negative affects low and high in motivational intensity. Psychological Science, 21(2), 211–215. https://doi.org/10.1177/0956797609359622
George, C., & West, M. (2001). The development and preliminary validation of a new measure of adult attachment: The Adult Attachment Projective. Attachment & Human Development, 3(1), 30–61. https://doi.org/10.1080/14616730010024771
Grandchamp, R., Braboszcz, C., & Delorme, A. (2014). Oculometric variations during mind wandering. Frontiers in Psychology, 5, 31. https://doi.org/10.3389/fpsyg.2014.00031
Harmon-Jones, E., Gable, P. A., & Price, T. F. (2012). The influence of affective states varying in motivational intensity on cognitive scope. Frontiers in Integrative Neuroscience, 6(73), 1–5. https://doi.org/10.3389/fnint.2012.00073
Harmon-Jones, E., Gable, P. A., & Price, T. F. (2013). Does negative affect always narrow and positive affect always broaden the mind? Considering the influence of motivational intensity on cognitive scope. Current Directions in Cognitive Science, 22(4), 301–307. https://doi.org/10.1177/0963721413481353
Harrison, N. A., Singer, T., Rotshtein, P., Dolan, R. J., & Critchley, H. D. (2006). Pupillary contagion: Central mechanisms engaged in sadness processing. Social Cognitive & Affective Neuroscience, 1, 5–17. https://doi.org/10.1093/scan/nsl006
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E. D., Robinson, B. S., Hodgson, D. J., & Inger, R. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ, 6, e4794. https://doi.org/10.7717/peerj.4794
Hoffmann, A., Ettinger, U., Del Paso, G. A. R., & Duschek, S. (2017). Executive function and cardiac autonomic regulation in depressive disorders. Brain & Cognition, 118, 108–117. https://doi.org/10.1016/j.bandc.2017.08.003
Hoffmann, A., Maran, T., & Sachse, P. (2020). How we perceive others resembling us. i-Perception. https://doi.org/10.1177/2041669520966623
Javela, J. J., Mercadillo, R. E., & Ramírez, J. M. (2008). Anger and associated experiences of sadness, fear, valence, arousal, and dominance evoked by visual scenes. Psychological Reports, 103(3), 663–681. https://doi.org/10.2466/pr0.103.3.663-681
Kreibig, S. D. (2010). Autonomic nervous system activity in emotion: A review. Biological Psychology, 84(3), 394–421. https://doi.org/10.1016/j.biopsycho.2010.03.010
Kreibig, S. D., Wilhelm, F. H., Roth, W. T., & Gross, J. J. (2007). Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology, 44(5), 787–806. https://doi.org/10.1111/j.1469-8986.2007.00550.x
Labek, K., Viviani, R., Gizewski, E. R., Verius, M., & Buchheim, A. (2016). Neural correlates of the appraisal of attachment scenes in healthy controls and social cognition—An fMRI study. Frontiers in Human Neuroscience, 10, 345. https://doi.org/10.3389/fnhum.2016.00345
Labek, K., Berger, S., Buchheim, A., Bosch, J., Spohrs, J., Dommes, L., Beschoner, P., Stingl, J. C., & Viviani, R. (2017). The iconography of mourning and its neural correlates: A functional neuroimaging study. Social Cognitive & Affective Neuroscience, 12(8), 1303–1313. https://doi.org/10.1093/scan/nsx058
Lang, P. J. (2010). Emotion and motivation: Toward consensus definitions and a common research purpose. Emotion Review, 2, 229–233. https://doi.org/10.1177/1754073910361984
Lang, P. J., & Bradley, M. M. (2010). Emotion and the motivational brain. Biological Psychology, 84, 437–450. https://doi.org/10.1016/j.biopsycho.2009.10.007
Lang, P. J., & Bradley, M. M. (2013). Appetitive and defensive motivation: Goal-directed or goal-determined? Emotion Review, 5, 230–234. https://doi.org/10.1177/1754073913477511
Maran, T., Sachse, P., & Furtner, M. (2015). From specificity to sensitivity: Affective states modulate visual working memory for emotional expressive faces. Frontiers in Psychology, 6, 1297. https://doi.org/10.3389/fpsyg.2015.01297
Maran, T., Sachse, P., Martini, M., Weber, B., Pinggera, J., Zugal, S., & Furtner, M. (2017). Lost in time and space: States of high arousal disrupt implicit acquisition of spatial and sequential context information. Frontiers in Behavioral Neuroscience, 11, 206. https://doi.org/10.3389/fnbeh.2017.00206
Maran, T., Furtner, M., Liegl, S., Kraus, S., & Sachse, P. (2019). In the eye of a leader: Eye-directed gazing shapes perceptions of leaders’ charisma. The Leadership Quarterly, 30(6), 101337. https://doi.org/10.1016/j.leaqua.2019.101337
Maran, T., Furtner, M., Liegl, S., Ravet-Brown, T., Haraped, L., & Sachse, P. (2020). Visual attention in real-world conversation: Gaze patterns are modulated by communication and group size. Applied Psychology. https://doi.org/10.1111/apps.12291
Mather, M., & Sutherland, M. R. (2011). Arousal-biased competition in perception and memory. Perspectives in Psychological Sciences, 6, 114–133. https://doi.org/10.1177/1745691611400234
Mather, M., Clewett, D., Sakaki, M., & Harley, C. W. (2016). GANEing traction: The broad applicability of NE hotspots to diverse cognitive and arousal phenomena. Behavior & Brain Sciences, 39, e228. https://doi.org/10.1017/s0140525x16000017
Miller, J. G., Nuselovici, J. N., & Hastings, P. D. (2016). Nonrandom acts of kindness: Parasympathetic and subjective empathic responses to sadness predict children’s prosociality. Child Development, 87(6), 1679–1690. https://doi.org/10.1111/cdev.12629
Nesse, R. M., & Ellsworth, P. C. (2009). Evolution, emotions, and emotional disorders. American Psychologist, 64(2), 129–139. https://doi.org/10.1037/a0013503
Overbeek, T. J. M., van Boxtel, A., & Westerink, J. H. D. M. (2012). Respiratory sinus arrhythmia responses to induced emotional states: Effects of RSA indices, emotion induction method, age, and sex. Biological Psychology, 91(1), 128–141. https://doi.org/10.1016/j.biopsycho.2012.05.011
Panayiotou, G. (2008). Emotional dimensions reflected in ratings of affective scripts. Personality and Individual Differences, 44(8), 1795–1806. https://doi.org/10.1016/j.paid.2008.02.006
Papa, A., & Maitoza, R. (2013). The role of loss in the experience of mourning: The case of job loss. Journal of Loss & Trauma, 18(2), 152–169. https://doi.org/10.1080/15325024.2012.684580
Pedrotti, M., Lei, S., Dzaack, J., & Rötting, M. (2011). A data-driven algorithm for offline pupil signal preprocessing and eyeblink detection in low-speed eye-tracking protocols. Behavior Research Methods, 43(2), 372–383. https://doi.org/10.3758/s13428-010-0055-7
Pieters, R., Wedel, M., & Batra, R. (2010). The stopping power of advertising: Measures and effects of visual complexity. Journal of Marketing, 74(5), 48–60. https://doi.org/10.1509/2Fjmkg.74.5.048
Piferi, R. L., Kline, K. A., Younger, J., & Lawler, K. A. (2000). An alternative approach for achieving cardiovascular baseline: Viewing an aquatic video. International Journal of Psychophysiology, 37(2), 207–217. https://doi.org/10.1016/S0167-8760(00)00102-1
Pijper, J., de Wied, M., van Rijn, S., van Goozen, S., Swaab, H., & Meeus, W. (2018). Executive attention and empathy-related responses in boys with oppositional defiant disorder or conduct disorder, with and without comorbid anxiety disorder. Child Psychiatry & Human Development, 49, 956–965. https://doi.org/10.1007/s10578-018-0810-z
Porges, S. W. (2007). The polyvagal perspective. Biological Psychology, 74(2), 116–143. https://doi.org/10.1016/j.biopsycho.2006.06.009
Prkachin, K. M., Williams-Avery, R. M., Zwaal, C., & Mills, D. E. (1999). Cardiovascular changes during induced emotion: An application of Lang’s theory of emotional imagery. Journal of Psychosomatic Research, 47, 255–267. https://doi.org/10.1016/S0022-3999(99)00036-7
Reichle, E. D., Reineberg, A. E., & Schooler, J. W. (2010). Eye movements during mindless reading. Psychological Science, 21, 1300–1310. https://doi.org/10.1177/0956797610378686
Salvi, C., & Bowden, E. M. (2016). Looking for creativity: Where do we look when we look for new ideas? Frontiers in Psychology, 7, 161. https://doi.org/10.3389/fpsyg.2016.00161
Salvi, C., Bricolo, E., Franconeri, S. L., Kounios, J., & Beeman, M. (2015). Sudden insight is associated with shutting out visual inputs. Psychonomic Bulletin & Review, 22(6), 1814–1819. https://doi.org/10.3758/s13423-015-0845-0
Schwabe, L. (2013). Stress and the engagement of multiple memory systems: Integration of animal and human studies. Hippocampus, 23, 1035–1043. https://doi.org/10.1002/hipo.22175
Schwabe, L. (2016). Memory under stress: From single systems to network changes. European Journal of Neuroscience, 45, 478–489. https://doi.org/10.1111/ejn.13478
Schwabe, L., & Wolf, O. T. (2013). Stress and multiple memory systems: From ‘thinking’ to ‘doing.’ Trends in Cognitive Science, 17, 60–68. https://doi.org/10.1016/j.tics.2012.12.001
Shear, M. K. (2015). Complicated mourning. The New England Journal of Medicine, 372(2), 153–160. https://doi.org/10.1056/NEJMcp1315618
Shear, M. K., Ghesquiere, A., & Glickman, K. (2013). Bereavement and complicated mourning. Current Psychiatry Reports, 15(11), 406. https://doi.org/10.1007/s11920-013-0406-z
Shi, H., Yang, L., Zhao, L., Su, Z., Mao, X., Zhang, L., & Liu, C. (2017). Differences of heart rate variability between happiness and sadness emotion states: A pilot study. Journal of Medical & Biological Engineering, 37(4), 527–539. https://doi.org/10.1007/s40846-017-0238-0
Shirai, M., & Suzuki, N. (2017). Is sadness only one emotion? psychological and physiological responses to sadness induced by two different situations: “loss of someone” and “failure to achieve a goal.” Frontiers in Psychology, 8, 288. https://doi.org/10.3389/fpsyg.2017.00288
Siegle, G. J., Steinhauer, S. R., Carter, C. S., Ramel, W., & Thase, M. E. (2003). Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy & Research, 27(3), 365–382. https://doi.org/10.1023/A:1023974602357
Sirois, S., & Brisson, J. (2014). Pupillometry. Wiley Interdisciplinary Reviews: Cognitive Science, 5(6), 679–692. https://doi.org/10.1002/wcs.1323
Smilek, D., Carriere, J. S. A., & Cheyne, J. A. (2010). Out of mind, out of sight eye blinking as indicator and embodiment of mind wandering. Psychological Science, 21, 786–789. https://doi.org/10.1177/2F0956797610368063
Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures, Boucsein, W., Fowles, D. C., Grimnes, S., Ben-Shakhar, G., Roth, W. T., Dawson, M., & Filion, D. L. (2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49(8), 1017–1034. https://doi.org/10.1111/j.1469-8986.2012.01384.x
Thayer, J. F., & Lane, R. D. (2007). The role of vagal function in the risk for cardiovascular disease and mortality. Biological Psychology, 74(2), 224–242. https://doi.org/10.1016/j.biopsycho.2005.11.013
Thayer, J. F., & Lane, R. D. (2009). Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews, 33(2), 81–88. https://doi.org/10.1016/j.neubiorev.2008.08.004
Thayer, J. F., Åhs, F., Fredrikson, M., Sollers, J. J., III., & Wager, T. D. (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience & Biobehavioral Reviews, 36(2), 747–756. https://doi.org/10.1016/j.neubiorev.2011.11.009
Uzzaman, S., & Joordens, S. (2011). The eyes know what you are thinking: Eye movements as an objective measure of mind wandering. Consciousness & Cognition, 20, 1882–1886. https://doi.org/10.1016/j.concog.2011.09.010
Vredeveldt, A., Hitch, G. J., & Baddeley, A. D. (2011). Eyeclosure helps memory by reducing cognitive load and enhancing visualisation. Memory & Cognition, 39(7), 1253–1263. https://doi.org/10.3758/s13421-011-0098-8
Granholm, E., & Steinhauer, S. R. (Eds.) (2004). Pupillometric measures of cognitive and emotional processes. International Journal of Psychophysiology, 52(1), 1–6. https://doi.org/10.1016/j.ijpsycho.2003.12.001
Tarvainen, M. P., Niskanen, J. P., Lipponen, J. A., Ranta-Aho, P. O., & Karjalainen, P. A. (2009). Kubios HRV—A software for advanced heart rate variability analysis. In 4th European conference of the international federation for medical and biological engineering (pp. 1022–1025). Springer.
Zugal, S., Pinggera, J., Neurauter, M., Maran, T., & Weber, B. (2017). Cheetah experimental platform web 1.0: cleaning pupillary data. arXiv preprint arXiv:1703.09468
Acknowledgements
We are grateful to Daniel Lauf and Stella Ilg for their help with ECG data pre-processing.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck. The Tyrolean research funding supported the study (TWF Project Number: 280567).
Author information
Authors and Affiliations
Contributions
AH contributed to study design, data analysis and interpretation, drafting and revising the manuscript; TM contributed to study design, data collection, drafting and revising the manuscript; SL and TG contributed to data collection, processing and preparation as well as drafting the manuscript; UL contributed to data processing and preparation; PS contributed to drafting and revising the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The following details are corrected in the article.
(a). The corresponding author “Alexandra Hoffmann” and co-author “Thomas Maran” affiliation has been corrected. Also, the co-author “Thomas Maran” e-mail address has been updated as “thomas.k.maran@gmail.com”.
(b). In Fig. 3 there is a mistake in the labeling of the graphics. The y-axis of Fig. 3C should be labelled as “Number of fixations” instead of “First fixation duration”. The Fig. 3 is corrected.
(c). On page 285, in the last sentence of the second paragraph the following sentence “Thus, our results only partially support our third by showing reduced HRV as a response to mourning stimuli, as they do not seem to be specific to mourning.” should read as “Thus, our results only partially support our third hypothesis by showing reduced HRV as a response to mourning stimuli, as they do not seem to be specific to mourning.”
(d). On page 287, in the last paragraph of the discussion the following sentence “While we replicated earlier findings of increased EDA (Davydov et al., 2011) and decreased HRV parameters (Overbeek et al., 2012), we were not able to prove this as a specific pattern for mourning stimuli only.” should read as “While we replicated earlier findings of decreased HRV parameters (Overbeek et al., 2012), we were not able to prove this as a specific pattern for mourning stimuli only.”
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hoffmann, A., Maran, T., Grünbaum, T. et al. A psychophysiological investigation of mourning: There are two sides to the story. Motiv Emot 46, 276–290 (2022). https://doi.org/10.1007/s11031-022-09928-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11031-022-09928-3