Abstract
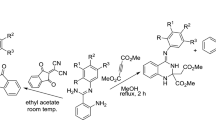
Various 2-[5-(aryl)-1,2,4-oxadiazol-3-yl]quinazolin-\(4(3H)\)-ones have been synthesized from the reaction of diaminoglyoxime-based nitrones with methyl 2-aminobenzoate or 2-aminobenzamide in the presence of acetic acid at \(100\,{^\circ }\mathrm{C}\). The reaction was extended as a one-pot three-component approach starting from diaminoglyoxime, aldehyde and methyl 2-aminobenzoate.
Graphical Abstract










Similar content being viewed by others
References
Kakanejadifard A (2004) A modified-one pot synthesis of diaminoglyoxime. Iran J Chem Chem Eng 23:117–118. doi:1021-9986/04/1/117
Andrianov VG, Eremeev AV (1990) \(\upsigma \)-Adducts in the 1,2,4-oxadiazole series. Chem Heterocycl Compd 26:714–714. doi:10.1007/BF00756439
Trusule M, Kupce E, Augustane I, Verovskii NV, Lukevics E, Baumane L, Gavars R, Stradins J (1991) Synthesis, antitumor activity, and electrochemical properties of furan-containing uracil derivatives. Khimiya Geterotsiklicheskikh Soedinenii 12:1687–1694
Moghimi A, Hosseinzadeh Khanmiri R, Shaabani A, Hamadani H (2013) A green synthesis of nitrones from diamino glyoxime using aldehydes and ketones. J Iran Chem Soc 10:929–936. doi:10.1007/s13738-013-0230-8
Moghimi A, hosseinzadeh Khanmiri R, Omrani I, Shaabani A (2013) A new library of 4(3H)- and 4,4(3H,3H)-quinazolinones and 2-(5-alkyl-1,2,4-oxadiazol-3-yl)quinazolin-4(3H)-one obtained from diaminoglyoxime. Tetrahedron Lett 54:3956–3959. doi:10.1016/j.tetlet.2013.05.065
Hosseinzadeh Khanmiri R, Moghimi A, Shaabani A, Valizadeh H, Ng SW (2014) Diaminoglyoxime as a versatile reagents in the synthesis of bis(1,2,4-oxadiazoles),1,2,4-oxadiazolyl-quinazolines and 1,2,4-oxadiazolyl-benzothiazinones. Mol Divers 18:769–774. doi:10.1007/s11030-014-9536-4
Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis (2008) Novel strategies in synthesis. In: Feuer H (ed) Chapter 2. Nitrones: novel strategies in synthesis (Igor Alexeevich Grigor’ev), 2nd edn. Wiley, Hoboken, pp 129–434
Gothelf KV, Jqrensen KA (1998) Asymmetric 1,3-dipolar cycloaddition reactions. Chem Rev 98:863–909. doi:10.1021/cr970324e
Coskun N, Tat FT (2003) Synthesis and ring opening reactions of tetrahydroimidazo[1,5-b][1,2,4]oxadiazol-2(1 H)-thiones. Phosphorus Sulfur Silicon Relat Elem 178:881–886. doi:10.1080/10426500307788
Maxim AV, Tikhon G Sh, Tatyana VR, Yury VG, Pervukhina Natalie V, Burdukov Aleksei B, Grigor’ev Igor A (2007) \(\upalpha \)-Organoelement nitrones: synthesis, properties, and IR and \(^{13}{\rm C}\) NMR spectral and X-ray structural characterization. Organometallics 26:1607–1615. doi:10.1021/om060883o
Avijit B, Piyali S (2001) Recent studies on 1,3-dipolar cycloadditions of nitrones. J Indian Inst Sci 81:313–323
Maxim AV, Igor AG, Leonid BV (2000) Dipole-stabilized carbanions in series of cyclic aldonitrones. Part 2: reactions of the metalated aldonitrones derivatives of 3-imidazoline 3-oxide and 2H-imidazole 1-oxide with aldehydes and ketones. Tetrahedron 56:4071–4077. doi:10.1016/S0040-4020(00)00321-5
Green AR, Ashwood T, Odergren T, Jackson DM (2003) Nitrones as neuroprotective agents in cerebral ischemia, with particular reference to NXY-059. Pharm Ther 100:195–214. doi:10.1016/j.pharmthera.2003.07.003
Tiemann F, Kruger P (1884) Ber Dtsch Chem Ges 17:1685–1698
Coskun N, Mert H, Arikan N (2006) Dipolar cycloadditions of imidazoline 3-oxides with N-arylmaleimides. Synthesis and diethylamine induced ring-opening of exo and endo hexahydro-7-oxa-2,5,6 a-triaza-cyclopenta[a]pentalene-1,3-diones. Tetrahedron 62:1351–1359. doi:10.1016/j.tet.2005.11.038
Xu Y, Shuo Zh, Yuanqiang W, Zemei G, Tieming Ch, Li Runtao (2012) Propylene oxide assisted one-pot, tandem synthesis of substituted-1,3,4-oxadiazole-2(3H)-ones in water. Tetrahedron 68:7978–7983. doi:10.1016/j.tet.2012.07.004
Hemming K (2008) In: Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK (eds) Comprehensive heterocyclic chemistry III, vol 5, 3rd edn. Elsevier, London, pp 243–248
Andersen KE, Lundt BF, Braestrup ASC (1996) Oxadiazoles as bioisosteric transformations of carboxylic functionalities. Eur J Med Chem 31:417–425. doi:10.1016/0223-5234(96)89169-0
Borgs S, Estenne-Bouhtou G, Luthman K, Csoregh I, Hesselink W, Hacksell U (1995) Synthesis of 1,2,4-oxadiazole-, 1,3,4-oxadiazole-, and 1,2,4-triazole-derived dipeptidomimetics. J Org Chem 60:3112–3120. doi:10.1021/jo00115a029
Buchanan JL, Vu CB, Merry TJ, Corpuz EG, Pradeepen SG, Mani UN, Yang M, Plake HR, Varkhedkar VM, Lynch BA, MacNeil IA, Loiacono KA, Tiong CL, Holt DA (1999) Structure-activity relationships of a novel class of Src \(\text{ SH }_{2}\) inhibitors. Bioorg Med Chem Lett 9:2359–2364. doi:10.1002/chin.199944218
Vu CB, Corpuz EG, Merry TJ, Pradeepan SG, Barlett C, Bohacek RS, Botfield MC, Eyermann CJ, Lynch BA, MacNeil IA, Ram MK, Schravendijk MR, Violette S, Sawyer TK (1999) Discovery of potent and selective \(\text{ SH }_{2}\) inhibitors of the tyrosine kinase ZAP-70. J Med Chem 42:4088–4098. doi:10.1021/jm990229t
Street LJ, Baker R, Book T, Kneen CO, MacLeod AM, Merchant KJ, Showell GA, Saunders J, Herbert RH, Freedman SB, Harley EA (1990) Synthesis and biological activity of 1,2,4-oxadiazole derivatives: highly potent and efficacious agonists for cortical muscarinic receptors. J Med Chem 33:2690. doi:10.1021/jm00172a003
Olga Adelfinskaya C, Vishal Nashine E, Donald BV, Jo D (2005) Efficient primer strand extension beyond oxadiazole carboxamide nucleobases. J Am Chem Soc 127:16000–16001. doi:10.1021/ja054226j
Diana GD, Volkots DL, Nitz TJ, Bailey TR, Long MA, Vescio N, Aldous S, Pevear DC, Dotko FJ (1994) Oxadiazoles as ester bioisosteric replacements in compounds related to disoxaril Antirhinovirus activity. J Med Chem 37:2421–2436. doi:10.1021/jm00041a022
Roppe J, Smith ND, Huang D, Tehrani L, Wang B, Anderson J, Brodkin J, Chung J, Jiang X, King C, Munoz B, Varney MA, Prasit P, Cosford NDP (2004) Discovery of novel heteroarylazoles that are metabotropic glutamate subtype 5 receptor antagonists with anxiolytic activity. J Med Chem 47:4645–4648. doi:10.1021/jm049828c
Swain CJ, Baker R, Kneen C, Moseley J, Saunders J, Seward EM, Stevenson G, Beer M, Stanton J, Watling K (1991) Novel 5-\(\text{ HT }_{3}\) antagonists Indole oxadiazoles. J Med Chem 34:140–151. doi:10.1021/jm00105a021
Li Z, Chen W, Hale JJ, Lynch CL, Mills SG, Hajdu R, Keohane CA, Rosenbach MJ, Milligan JA, Shei GJ, Chrebet G, Parent SA, Bergstrom J, Card D, Forrest M, Quackenbush EJ, Wickham LA, Vargas H, Evans RM, Rosen H, Mandala S (2005) Discovery of potent 3,5-diphenyl-1,2,4-oxadiazole sphingosine-1-phosphate-1 (S1P1) receptor agonists with exceptional selectivity against S1P2 and S1P3. J Med Chem 48:6169–6173. doi:10.1021/jm0503244
Murali MG, Naveen P, Udayakumar D, Vandana Y, Ritu S (2012) Synthesis and characterization of thiophene and fluorene based donor-acceptor conjugated polymer containing 1,3,4-oxadiazole units for light-emitting diodes. Tetrahedron Lett 53:157–161. doi:10.1016/j.tetlet.2011.10.157
Rice K, Nuss JM (2001) An improved synthesis of 1,2,4-oxadiazoles on solid support. Bioorg Med Chem Lett 11:753–757. doi:10.1016/S0960-894X(01)00028-2
Gangloff AR, Litvak J, Shelton EJ, Sperandio D, Wang VR, Rice KD (2001) Synthesis of 3,5-disubstituted-1,2,4-oxadiazoles using tetrabutylammonium fluoride as a mild and efficient catalyst. Tetrahedron Lett 42:1441–1443. doi:10.1016/j.tet.2009.11.079
Hamz’e A, Hernandez JF, Fulcrand P, Martimnez J (2003) Synthesis of various 3-substituted 1,2,4-oxadiazole-containing chiral beta 3- and alpha-amino acids from Fmoc-protected aspartic acid. J Org Chem 68:7316–7321. doi:10.1021/jo0345953y
Bhaskar CD, Xiang-Ying T, Patrick R, Todd E (2012) Design and synthesis of 3,5-disubstituted boron-containing 1,2,4-oxadiazoles as potential combretastatin A-4 (CA-4) analogs. Tetrahedron Lett 53:3947–3950. doi:10.1016/j.tetlet.2012.02.110
Alafeefy AM (2008) Synthesis and antimicrobial activity of some new quinazolin-4(3H)-one. Pharm Biol 46:751–756. doi:10.1080/13880200802315907
Sen Gupta AK, Misra HK (1980) Synthesis and evaluation of substituted quinazolone derivatives for antibacterial, antifungal, and antiacetylcholinesterase activities. J Pharm Sci 69:1313–1317. doi:10.1002/jps.2600691120
Alagarsamy V, Pathak US (2007) Synthesis and antihypertensive activity of novel 3-benzyl-2-substituted-3H-[1,2,4]triazolo[5,1-b]quinazolin-9-ones. Bioorg Med Chem 15:3457–3462. doi:10.7324/JAPS.2013.30331
Jessy EM, Sambanthan AT, Alex J, Sridevi CH, Srinivasan KK (2007) Synthesis and biological evaluation of some novel quinazolones. Ind J Pharm Sci 69:476–478. doi:10.4103/0250-474X.34571
Shukla JS, Agarwal K, Rastogi R (1983) Synthesis of some new 2-substituted 3-[4-(N’-arylsulphonylbiguanido)phenyl]quinazolin-4-one hydrochlorides as potential anthelminthic agents. Arch Pharm 316:525–529. doi:10.1002/ardp.19833160610
Khalil AA, Abdel Hamide SG, Al-Obaid AM, El-Subbagh HI (2003) Substituted quinazolines, part 2. Synthesis and in-vitro anticancer evaluation of new 2-substituted mercapto-3H-quinazoline analogs. Arch Pharm 336:95–103. doi:10.1002/ardp.200390011
Laddha SS, Bhatnagar SP (2008) Rapid microwave-assisted solution phase synthesis of 6,8-disubstituted 2-phenyl-3-(substituted- benzothiazol-2-yl)-4-[3H]-quinazolinones as novel anticonvulsants. Phosphorus Sulfur Silicon Relat Elem 183:2262–2273. doi:10.1080/10426500801957766
Qiuping D, Xianjin L, Jinsheng Y, Qiulan Z, Dan W, Banpeng C, Yiyuan P (2012) Access to functionalized 4-benzylidene-4H-benzo[d][1,3]thiazines via tandem addition-cyclization/cross-coupling reactions. Tetrahedron 68:3937–3941. doi:10.1016/j.tet.2012.03.098
Alagarsamy V, Murugesan S, Dhanabal K, Murugan M, Clercq E (2007) AntiHIV, antibacterial and antifungal activities of some novel 2-methyl-3-(substituted methylamino)-(3H)-quinazolin-4-ones. Ind J Pharm Sci 69:304–307. doi:10.4103/0250-474X.33167
Raffa D, Daidone G, Maggio B, Schillaci D, Plescia F (1999) Synthesis and antiproliferative activity of novel 3-(indazol-3-yl)-quinazolin-4(3H)-one and 3-(indazol-3-yl)-benzotriazin-4(3H)-one derivatives. Arch Pharm 332:317–320. doi:10.1002/(SICI)1521-4184(19999)332:9<317:AID-ARDP317>3.0.CO;2-R
Mosaad SM, Mohammed KI, Ahmed MA, Abdel-Hamide SG (2004) Synthesis of certain new 6-iodoquinazolines as potential antitubercular agents. J Appl Sci 4:302–307. doi:10.3923/jas.2004.302.307
Navin BP, Jaymin CP (2010) Synthesis and antimicrobial activity of novel 1,3,4-oxadiazolyl-quinazolin-4(3H)ones. J Heterocycl Chem 47:923–931. doi:10.3797/scipharm.0912-16
Raffa D, Edler MC, Daidone G, Maggio B, Merickech M, Plescia S, Schillaci D, Bai R, Hamel E (2004) Synthesis, cytotoxicity, and inhibitory effects on tubulin polymerization of a new 3-heterocyclo substituted 2-styrylquinazolinones. Eur J Med Chem 39:299–304. doi:10.1016/j.ejmech.12.009
Maarouf AR, El-Bendary ER, Goda FE (2004) Synthesis and evaluation of some novel quinazolinone derivatives as diuretic agents. Arch Pharm 337:527–532. doi:10.1002/jps.2600691120
Kurogi Y, Inoue Y, Tsutsumi K, Nakamura S, Nagao K, Yoshitsugu H, Tsuda Y (1996) Synthesis and antiproliferativeactivities of novel 2-[4-[diethoxyphosphoryl)methyl]phenyl]quinazolines and 4(3H)-quinazolinones. J Med Chem 39:1433–1437. doi:10.1002/(SICI)1521-4184(19999)332:9<317:AID-ARDP317>3.0.CO;2-R
Sorra K, Mukkanti K, Pusuluri S (2012) Palladium catalyzed synthesis of quinazolino [1,4] benzodiazepine alkaloids and analogous. Tetrahedron 68:2001–2006. doi:10.1016/j.tet.2011.12.032
Shaabani A, Rezayan A, Keshipour S, Sarvary A, Ng SW (2009) A novel one-pot three-(in situ five-)component condensation reaction: an unexpected approach for the synthesis of tetrahydro-2,4-dioxo-1H-benzo[b][1,5]diazepine-3-yl-2-methylpropanamide derivatives. Org Lett 11:3342–3345. doi:10.1021/ol901196zy
Shaabani A, Maleki A, Moghimi-Rad J (2007) A novel isocyanide-based three-component reaction: synthesis of highly substituted 1,6-dihydropyrazine-2,3-dicarbonitrile derivatives. J Org Chem 72:6309–6311. doi:10.1021/jo0707131
Shaabani A, Maleki A, Mofakham H, Moghimi- Rad J (2008) A novel one-pot pseudo-five-component synthesis of 4,5,6,7-tetrahydro-1H-1,4-diazepine-5-carboxamide derivatives. J Org Chem 73:3925–3927. doi:10.1021/jo00930a004
Acknowledgments
We gratefully acknowledge financial support from the Research Council of Imam Hossein and Shahid Beheshti Universities. We also wish to thank the Ministry of Higher Education of Malaysia (Grant No. UM.C/625/1/HIR/247) for supporting this study.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hosseinzadeh-Khanmiri, R., Moghimi, A., Shaabani, A. et al. Synthesis of 2-(1,2,4-oxadiazol-3-yl)quinazolin-4(3H)-ones from diaminoglyoxime-based nitrones. Mol Divers 19, 501–510 (2015). https://doi.org/10.1007/s11030-015-9588-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-015-9588-0