Abstract
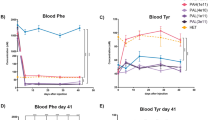
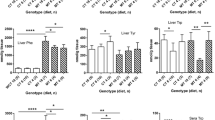
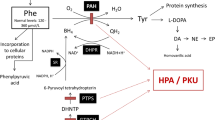
To gain insight into the potential protective mechanisms of low phenylalanine diet (LPD) in phenylketonuria (PKU), gene expression profiles were studied in the cerebral cortex and hippocampus of a PKU mouse model (BTBR-Pahenu2). PKU mice were fed with low Phe diet (LPD-PKU group) and normal diet (PKU group). Wild-type mice were treated with normal diet (WT group) as control. After 12 weeks, we detected gene expression in the cerebral cortex and hippocampus of the three groups by RNA-sequencing, and then screened the differentially-expressed genes (DEGs) among the groups by bioinformatics analyses. We found that the transcriptional profiles of both cerebral cortex and hippocampus changed markedly between PKU and WT mice. Furthermore, LPD changed the transcriptional profiles of the cerebral cortex and the hippocampus of PKU mice significantly, especially in the cerebral cortex, with overlaps of genes that changed with the disease and altered by LPD treatment. In the cerebral cortex, hundreds of DEGs enriched in a wide spectrum of biological processes, molecular function, and cellular component, including nervous system development, axon development and guidance, calcium ion binding, modulation of chemical synaptic transmission, and regulation of protein kinase activity. In the hippocampus, the overlapping genes were enriched in positive regulation of long term synaptic, negative regulation of excitatory postsynaptic potential, positive regulation of synapse assembly. Our results showed that genes impaired in PKU and then rescued by LPD might indicate the potential protective capability of LPD in the PKU brain.





Similar content being viewed by others
Data availability
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
Al Hafid N, Christodoulou J (2015) Phenylketonuria: a review of current and future treatments. Transl Pediatr 4(4):304–317
Battaglia-Mayer A (2019) A brief history of the encoding of hand position by the cerebral cortex: implications for motor control and cognition. Cereb Cortex 29(2):716–731
Bayat A, Møller LB, Lund AM (2015) Diagnostics and treatment of phenylketonuria. Ugesk Laeger 177(8):V07140383
Blau N (2016) Genetics of phenylketonuria: then and now. Hum Mutat 37(6):508–515
Blau N, van Spronsen FJ, Levy HL (2010) Phenylketonuria. Lancet 376(9750):1417–1427
Bortoluzzi VT, de Franceschi ID, Rieger E, Wannmacher CM (2014) Co-administration of creatine plus pyruvate prevents the effects of phenylalanine administration to female rats during pregnancy and lactation on enzymes activity of energy metabolism in cerebral cortex and hippocampus of the offspring. Neurochem Res 39(8):1594–1602
Collado-Torres L, Burke EE, Peterson A, Shin J, Straub RE, Rajpurohit A, Semick SA, Ulrich WS, BrainSeq Consortium, Price AJ, Valencia C, Tao R, Deep-Soboslay A, Hyde TM, Kleinman JE, Weinberger DR, Jaffe AE (2019) Regional heterogeneity in gene expression, regulation, and coherence in the frontal cortex and Hippocampus across development and schizophrenia. Neuron. 103(2):203–216
Couce ML, Sánchez-Pintos P, Vitoria I, De Castro MJ, Aldámiz-Echevarría L, Correcher P, Fernández-Marmiesse A, Roca I, Hermida A, Martínez-Olmos M, Leis R (2018) Carbohydrate status in patients with phenylketonuria. Orphanet J Rare Dis. 13(1):103
Dyer CA, Kendler A, Philibotte T, Gardiner P, Cruz J, Levy HL (1996) Evidence for central nervous system glial cell plasticity in phenylketonuria. J Neuropathol Exp Neurol 55(7):795–814
Ferreira BK, Rodrigues MT, Streck EL, Ferreira GC, Schuck PF (2021) White matter disturbances in phenylketonuria: possible underlying mechanisms. J Neurosci Res 99(1):349–360
González MJ, Gassió R, Artuch R, Campistol J (2016) Impaired neurotransmission in early-treated phenylketonuria patients. Semin Pediatr Neurol 23(4):332–340
Güttler F, Lou H (1986) Dietary problems of phenylketonuria: effect on CNS transmitters and their possible role in behaviour and neuropsychological function. J Inherit Metab Dis 9(Suppl 2):169–177
Hofman DL, Champ CL, Lawton CL, Henderson M, Dye L (2018) A systematic review of cognitive functioning in early treated adults with phenylketonuria. Orphanet J Rare Dis 13(1):150
Hörster F, Schwab MA, Sauer SW, Pietz J, Hoffmann GF, Okun JG, Kölker S, Kins S (2006) Phenylalanine reduces synaptic density in mixed cortical cultures from mice. Pediatr Res 59(4 Pt 1):544–548
Kappel DB, Schuch JB, Rovaris DL, da Silva BS, Müller D, Breda V, Teche SP, Riesgo SR, Schüler-Faccini L, Rohde LA, Grevet EH, Bau CHD (2019) ADGRL3 rs6551665 as a common vulnerability factor underlying attention-deficit/hyperactivity disorder and autism Spectrum disorder. NeuroMolecular Med 21(1):60–67
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T (2014) Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet 46(7):678–684
Leuzzi V, Chiarotti F, Nardecchia F, van Vliet D, van Spronsen FJ (2020) Predictability and inconsistencies of cognitive outcome in patients with phenylketonuria and personalised therapy: the challenge for the future guidelines. J Med Genet 57(3):145–150
Lisman J, Buzsáki G, Eichenbaum H, Nadel L, Ranganath C, Redish AD (2017) Viewpoints: how the hippocampus contributes to memory, navigation and cognition. Nat Neurosci 20(11):1434–1447
Luengo-Sanchez S, Fernaud-Espinosa I, Bielza C, Benavides-Piccione R, Larrañaga P, DeFelipe J (2018) 3D morphology-based clustering and simulation of human pyramidal cell dendritic spines. PLoS Comput Biol 14(6):e1006221
Maggio N, Vlachos A (2014) Synaptic plasticity at the interface of health and disease: new insights on the role of endoplasmic reticulum intracellular calcium stores. Neuroscience. 281:135–146
McDonald JD, Charlton CK (1997) Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 39(3):402–405
Mortuaire G, Marchetti P, Formstecher P, Danzé PM (2004) Micro-array based technologies to study the proteome: technological progress and applications. Ann Biol Clin 62(2):139–148
Nardecchia F, Manti F, Chiarotti F, Carducci C, Carducci C, Leuzzi V (2015) Neurocognitive and neuroimaging outcome of early treated young adult PKU patients: a longitudinal study. Mol Genet Metab 115(2–3):84–90
Palermo L, Geberhiwot T, MacDonald A, Limback E, Hall SK, Romani C (2017) Cognitive outcomes in early-treated adults with phenylketonuria (PKU): a comprehensive picture across domains. Neuropsychology. 31(3):255–267
Pchitskaya E, Bezprozvanny I (2020) Dendritic spines shape analysis-classification or Clusterization? Perspective. Front Synaptic Neurosci 12:31
Pereira AC, Gray JD, Kogan JF, Davidson RL, Rubin TG, Okamoto M, Morrison JH, McEwen BS (2017) Age and Alzheimer's disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry 22(2):296–305
Qin M, Smith CB (2007) Regionally selective decreases in cerebral glucose metabolism in a mouse model of phenylketonuria. J Inherit Metab Dis 30(3):318–325
Rocha JC, MacDonald A (2016) Dietary intervention in the management of phenylketonuria: current perspectives. Pediatric Health Med Ther 7:155–163
Rocha-Dias PF, Simao-Silva DP, Silva SSLD, Souza RKM, Darreh-Shori T, Furtado-Alle L, Souza RLR (2020) Influence of a genetic variant of CHAT gene over the profile of plasma soluble ChAT in Alzheimer disease. Genet Mol Biol 43(4):e20190404
Shedlovsky A, McDonald JD, Symula D, Dove WF (1993) Mouse models of human phenylketonuria. Genetics. 134(4):1205–1210
Streck EL, Edom PT, Noriler ME, Borges LF, Pontes ZL, Parolo E, Dutra-Filho CS, Wannmacher CM, Wyse AT (2000) Effect of phenylalanine and p-chlorophenylalanine on Na+, K+-ATPase activity in the synaptic plasma membrane from the cerebral cortex of rats. Metab Brain Dis 15(2):105–114
Takeo YH, Shuster SA, Jiang L, Hu MC, Luginbuhl DJ, Rülicke T, Contreras X, Hippenmeyer S, Wagner MJ, Ganguli S, Luo L (2021 Feb 17) GluD2- and Cbln1-mediated competitive interactions shape the dendritic arbors of cerebellar Purkinje cells. Neuron. 109(4):629–644
Vardy ERLC, MacDonald A, Ford S, Hofman DL (2020) Phenylketonuria, co-morbidity, and ageing: a review. J Inherit Metab Dis 43(2):167–178
Zhang Y, Gu X, Yuan X (2007) Phenylalanine activates the mitochondria-mediated apoptosis through the RhoA/rho-associated kinase pathway in cortical neurons. Eur J Neurosci 25(5):1341–1348
Funding
This work was supported by Shanghai Municipal Health Commission (No.202040448) and the National Natural Science Foundation of China (No.81600701).
Author information
Authors and Affiliations
Contributions
LL and XG conceived and designed the study. SH, TZ and SZ prepared an analytical plan, analyzed data, and drafted the initial manuscript. SH and LL collaborated in the revision and interpretation of the data and results, and revised the manuscript. SH, TZ, SZ involved in data collection, manuscript review and revision, XZ and FX contributed to the detection of the blood Phe concentration, the management of animals and tissue extraction. All authors commented on the manuscript and approved the final manuscript as submitted.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures were carried out in accordance with the National Regulations on the Administmiceion of Labomiceory Animals. Ethical approval was obtained from the Ethics Committee of Xinhua Hospital.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hong, S., Zhu, T., Zheng, S. et al. Gene expression profiles in the brain of phenylketonuria mouse model reversed by the low phenylalanine diet therapy. Metab Brain Dis 36, 2405–2414 (2021). https://doi.org/10.1007/s11011-021-00818-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11011-021-00818-0