Abstract
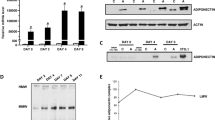
Adiponectin (ADN) is an abundant protein in serum, secreted by adipocytes, that acts as a signal for fat metabolism. It is marked by a complex molecular structure that results from processes within the secretory pathway, producing a canonical set of multimers. ADN may also be secreted from cardiomyocytes, where a unique sarcomeric endoplasmic/sarcoplasmic reticulum (ER/SR) substructure has been characterized primarily for its Ca handling. We expressed ADN in cultured primary adult cardiomyocytes and nonmuscle (COS) cells. After 48 h of ADN expression by adenovirus treatment, roughly half of synthesized ADN was secreted from cardiomyocytes, and half was still in-transit within inner membrane compartments, similar to COS cells. Cardiomyocytes and COS cells both produced ADN in the three canonical forms: trimers, hexamers, and 18-mers. Higher rates of secretion occurred for higher-molecular weight multimers, especially 18-mers. The highest levels of ADN protein, whether in transit or secreted, were present as trimers and hexamers. In nonmuscle cell lines, ADN trafficked through ER and Golgi compartments as expected. In contrast, ADN in primary adult cardiomyocytes populated ER/SR tubules along the edges of sarcomeres that emanated from nuclear surfaces. Prominent co-localization of ADN occurred with calsequestrin, a marker of junctional SR, the Ca2+-release compartment of the cell. The early steps in ADN trafficking re-trace those recently described for newly made junctional SR proteins, involving a nuclear envelope (NE) translocation into SR tubules that are oriented along sarcolemmal transverse (T)-tubules (NEST pathway).











Similar content being viewed by others
Abbreviations
- ER:
-
Endoplasmic reticulum
- jSR:
-
Junctional sarcoplasmic reticulum
- ADN:
-
Adiponectin
- MOI:
-
Multiplicity of infection
- Pfu:
-
Plaque-forming units
- HMW:
-
High molecular weight
- MMW:
-
Medium molecular weight
- LMW:
-
Low molecular weight
- ADN-WT:
-
Wild-type (human) adiponectin
- ADN-Flag:
-
Flag-tagged adiponectin
- native-PAGE:
-
Native polyacrylamide gel electrophoresis
- BFA:
-
Brefeldin A
- NEST pathway:
-
Nuclear envelope to SR along T-tubules pathway
- z-tubules:
-
SR tubules that traffic newly made proteins roughly aligned with the sarcomeric z-line and T-tubules
References
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749
Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703
Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221:286–289. https://doi.org/10.1006/bbrc.1996.0587
Fang H, Judd RL (2018) Adiponectin regulation and function. Compr Physiol 8:1031–1063. https://doi.org/10.1002/cphy.c170046
Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo MS, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63. https://doi.org/10.1038/nm.2277
Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T (2003) Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem 278:40352–40363. https://doi.org/10.1074/jbc.M300365200
Richards AA, Stephens T, Charlton HK, Jones A, Macdonald GA, Prins JB, Whitehead JP (2006) Adiponectin multimerization is dependent on conserved lysines in the collagenous domain: evidence for regulation of multimerization by alterations in posttranslational modifications. Mol Endocrinol 20:1673–1687. https://doi.org/10.1210/me.2005-0390
Liu M, Liu F (2014) Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab 28:25–31. https://doi.org/10.1016/j.beem.2013.06.003
Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE (2008) Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149:2270–2282. https://doi.org/10.1210/en.2007-1561
Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, Heuser JE, Lodish HF (2003) Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem 278:50810–50817. https://doi.org/10.1074/jbc.M309469200
Suzuki S, Wilson-Kubalek EM, Wert D, Tsao TS, Lee DH (2007) The oligomeric structure of high molecular weight adiponectin. FEBS Lett 581:809–814. https://doi.org/10.1016/j.febslet.2007.01.046
Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE (2003) Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem 278:9073–9085. https://doi.org/10.1074/jbc.M207198200
Wang Y, Xu A, Knight C, Xu LY, Cooper GJ (2002) Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 277:19521–19529. https://doi.org/10.1074/jbc.M200601200
Amin RH, Mathews ST, Alli A, Leff T (2010) Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARgamma-dependent autocrine mechanism. Am J Physiol Heart Circ Physiol 299:H690–H698. https://doi.org/10.1152/ajpheart.01032.2009
Ding G, Qin Q, He N, Francis-David SC, Hou J, Liu J, Ricks E, Yang Q (2007) Adiponectin and its receptors are expressed in adult ventricular cardiomyocytes and upregulated by activation of peroxisome proliferator-activated receptor gamma. J Mol Cell Cardiol 43:73–84. https://doi.org/10.1016/j.yjmcc.2007.04.014
Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K (2004) Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med 10:1384–1389. https://doi.org/10.1038/nm1137
Guo B, Li Y, Jin X, Liu S, Miao C (2017) Nitric oxide/cyclic GMP pathway mediates the endothelin-1-upregulation of adiponectin expression in rat cardiomyocytes. Biomed Rep 7:267–271. https://doi.org/10.3892/br.2017.953
McFarland TP, Milstein ML, Cala SE (2010) Rough endoplasmic reticulum to junctional sarcoplasmic reticulum trafficking of calsequestrin in adult cardiomyocytes. J Mol Cell Cardiol:556–564
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Hendricks GL IIIrd, Hadley JA, Krzysik-Walker SM, Prabhu KS, Vasilatos-Younken R, Ramachandran R (2009) Unique profile of chicken adiponectin, a predominantly heavy molecular weight multimer, and relationship to visceral adiposity. Endocrinology 150:3092–3100. https://doi.org/10.1210/en.2008-1558
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD (1989) Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56:801–813
Klausner RD, Donaldson JG, Lippincott-Schwartz J (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116:1071–1080
Milstein ML, Houle TD, Cala SE (2009) Calsequestrin isoforms localize to different ER subcompartments: evidence for polymer and heteropolymer-dependent localization. Exp Cell Res 315:523–534. https://doi.org/10.1016/j.yexcr.2008.11.006
Gomez-Navarro N, Miller E (2016) Protein sorting at the ER-Golgi interface. J Cell Biol 215:769–778. https://doi.org/10.1083/jcb.201610031
Lippincott-Schwartz J, Roberts TH, Hirschberg K (2000) Secretory protein trafficking and organelle dynamics in living cells. Annu Rev Cell Dev Biol 16:557–589
Xie L, Boyle D, Sanford D, Scherer PE, Pessin JE, Mora S (2006) Intracellular trafficking and secretion of adiponectin is dependent on GGA-coated vesicles. J Biol Chem 281:7253–7259. https://doi.org/10.1074/jbc.M511313200
Sleiman NH, McFarland TP, Jones LR, Cala SE (2015) Transitions of protein traffic from cardiac ER to junctional SR. J Mol Cell Cardiol 29:35–45
Jorgensen AO, Shen AC, Daly P, MacLennan DH (1982) Localization of Ca2+ + Mg2+-ATPase of the sarcoplasmic reticulum in adult rat papillary muscle. J Cell Biol 93:883–892
Sommer JR, Jennings RB (1986) Ultrastructure of cardiac muscle. In: Fozzard HA (ed) The heart and cardiovascular system. Raven Press, New York, pp 61–100
Cala SE, Jones LR (1983) Rapid purification of calsequestrin from cardiac and skeletal muscle sarcoplasmic reticulum vesicles by Ca2+-dependent elution from phenyl- sepharose. J Biol Chem 258:11932–11936
Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523. https://doi.org/10.1038/ncb1404
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769. https://doi.org/10.1038/nature01705
van Andel M, Heijboer AC, Drent ML (2018) Adiponectin and Its Isoforms in Pathophysiology. Adv Clin Chem 85:115–147. https://doi.org/10.1016/bs.acc.2018.02.007
Wang ZV, Scherer PE (2016) Adiponectin, the past two decades. J Mol Cell Biol 8:93–100. https://doi.org/10.1093/jmcb/mjw011
Nilsson I, Kelleher DJ, Miao Y, Shao Y, Kreibich G, Gilmore R, von Heijne G, Johnson AE (2003) Photocross-linking of nascent chains to the STT3 subunit of the oligosaccharyltransferase complex. J Cell Biol 161:715–725. https://doi.org/10.1083/jcb.200301043
Nikonov AV, Hauri HP, Lauring B, Kreibich G (2007) Climp-63-mediated binding of microtubules to the ER affects the lateral mobility of translocon complexes. J Cell Sci 120:2248–2258. https://doi.org/10.1242/jcs.008979
Kaisto T, Metsikko K (2003) Distribution of the endoplasmic reticulum and its relationship with the sarcoplasmic reticulum in skeletal myofibers. Exp Cell Res 289:47–57
Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR (1997) Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem 272:23389–23397
Scriven DR, Dan P, Moore ED (2000) Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J 79:2682–2691
Sommer JR, Waugh RA (1976) The ultrastructure of the mammalian cardiac muscle cell–with special emphasis on the tubular membrane systems. A review. Am J Pathol 82:192–232
Bers DM, Shannon TR (2013) Calcium movements inside the sarcoplasmic reticulum of cardiac myocytes. J Mol Cell Cardiol 58:59–66. https://doi.org/10.1016/j.yjmcc.2013.01.002
Acknowledgements
This work was supported by an Incubator Grant 1-77311 from the Office of Vice President for Research, Wayne State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solarewicz, J., Manly, A., Kokoszka, S. et al. Adiponectin secretion from cardiomyocytes produces canonical multimers and partial co-localization with calsequestrin in junctional SR. Mol Cell Biochem 457, 201–214 (2019). https://doi.org/10.1007/s11010-019-03524-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-019-03524-9