Abstract
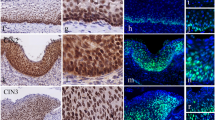
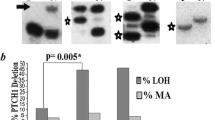
In this study, importance of Wnt-β-catenin pathway in the development of uterine cervical carcinoma was evaluated. For this purpose, the profiles (expression/methylation/deletion) of β-catenin, p-β-catenin (Y654), Wnt3a, and APC were studied in disease free normal cervical epithelium (n = 9), adjacent normal cervical epithelium of primary tumors (n = 70), CIN (n = 28), CACX (n = 102) samples, and two CACX cell lines (HeLa and SiHa). Immunohistochemical analysis revealed high/medium (74–95%) expression of β-catenin/p-β-catenin (Y654) and Wnt3a and low expression (23–26%) of APC in proliferating basal–parabasal layers contrary to differentiated spinous layer in normal cervix irrespective of HPV16 infection. The expression profile of the genes in the basal–parabasal layers did not change significantly during development of CACX. High (66%) promoter methylation of APC was seen in basal–parabasal layers and the cervical lesions (42–69%), unlike in spinous layers (25%). The promoter methylation status of APC was validated by in vitro demethylation experiments using 5-aza-dC in CACX cell lines. However, additional deletion of APC was significantly increased from CIN (12%) to stage I/II (40%) and became comparable in stage III/IV (48%) of the tumor. Patients with alterations (deletion/methylation) of APC and high/medium expression of Wnt3a/β-catenin/p-β-catenin (Y654) showed significantly poor survival. Thus our data indicate that cumulative effect of Wnt3a overexpression and APC inactivation are needed for overexpression of β-catenin during the development of CACX.







Similar content being viewed by others
References
Arbyn M, Castellsague X, de Sanjose S, Bruni L, Saraiya M, Bray F, Ferlay J (2008) Worldwide burden of cervical cancer in 2008. Ann Oncol 22:2675–2686
WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Related Diseases report in India. Summary Report. 2014
Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV et al (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 348:518–527
Snijders PJ, Steenbergen RD, Heideman DA, Meijer CJ (2006) HPV-mediated cervical carcinogenesis: concepts and clinical implications. J Pathol 208:152–164
Lopez J, Ruiz G, Organista-Nava J, Gariglio P, Garcia-Carranca A (2012) Human papillomavirus infections and cancer stem cells of tumors from the uterine cervix. Open Virol J 6:232–240
Chakraborty C, Dutta S, Mukherjee N, Samadder S, Roychowdhury A, Roy A, Mondal RK, Basu P, Roychoudhury S, Panda CK (2015) Inactivation of PTCH1 is associated with the development of cervical carcinoma: clinical and prognostic implication. Tumour Biol 36:1143–1154
Uhlen M et al (2005) A human protein atlas for normal and human cancer tissues based on antibody proteomics. Mol Cell Proteom 4(12):1920–1932 (Human protein atlas: Release version 15, www.proteinatlas.org)
Zhang Yanna, Liu Bangzhong, Zhao Qingyu, Hou Teng, Huang Xin (2014) Nuclear localization of β-catenin is associated with poor survival and chemo-/radioresistance in human cervical squamous cell cancer Int J. Clin Exp Pathol 7:3908–3917
Pesterel Hadce Elf, Erdoan Gülgün, Fimfiek Tayyp, Karavel Fieyda (2006) Cadherin/catenin expression in squamous lesions of uterine cervix. Turk J Cancer 36(2):064–068
MacDonald BT, Tamai K, He X (2009) Wnt/beta-catenin signaling: components, mechanisms, and disease. Dev Cell 17:9–26
Zi David, Ren N, Yang YJ (2015) Promoter methylation of APC gene in cervical cancer and precancerous lesions and its association with APC expression. J Pract Oncol 3:320–324
Van Veelen W, Le NH, Helvensteijn W, Blonden L, Theeuwes M, Bakker ER, Franken PF, van Gurp L, Meijlink F, van der Valk MA, Kuipers EJ, Fodde R, Smits R (2011) β-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut 9:1204–1212
Dasgupta S, Mukherjee N, Roy S, Roy A, Sengupta A, Roychowdhury S, Panda CK (2002) Mapping of the candidate tumor suppressor genes’ loci on human chromosome 3 in head and neck squamous cell carcinoma of an Indian patient population. Oral Oncol 38:6–15
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Singh RK, Maulik S, Mitra S, Mondal RK, Basu PS, Roychowdhury S, Panda CK (2006) Human papillomavirus prevalence in postradiotherapy uterine cervical carcinoma patients: correlation with recurrence of the disease. Int J Gynecol Cancer 16:1048–1054
Chakraborty C, Roychowdhury A, Samadder S, Roy A, Mandal RK, Basu P, Roychoudhury S, Panda CK (2016) Association of P16-RBSP3 inactivation with phosphorylated-RB1 over-expression in basal–parabasal layers of normal-cervix unchanged during CACX development. Biochem J 473:3221–3236
Mukherjee N, Bhattacharya N, Alam N, Roy A, Roychoudhury S, Panda CK (2012) Subtype-specific alterations of the Wnt signaling pathway in breast cancer: clinical and prognostic significance. Cancer Sci 103:210–220
Perrone F, Suardi S, Pastore E, Casieri P, Orsenigo M, Caramuta S, Dagrada G, Losa M, Licitra L, Bossi P et al (2006) Molecular and cytogenetic subgroups of oropharyngeal squamous cell carcinoma. Clin Cancer Res 12:6643–6651
Loginov VI, Maliukova AV, Seregin IuA, Khodyrev DS, Kazubskaia TP, Ermilova VD, Gar’kavtseva RF, Kiselev LL, Zabarovskii ER, Braga EA (2004) Methylation of the promoter region of the RASSF1A gene, a candidate tumor suppressor, in primary epithelial tumors. Molekuliarnaia biologiia 38:654–667
Ivanova T, Petrenko A, Gritsko T, Vinokourova S, Eshilev E, Kobzeva V, Kisseljov F, Kisseljova N (2002) Methylation and silencing of the retinoic acid receptor-beta 2 gene in cervical cancer. BMC Cancer 2:4
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Maiti GP, Mondal P, Mukherjee N, Ghosh A, Ghosh S, Dey S, Chakrabarty J, Roy A, Biswas J, Roychoudhury S, Panda CK (2013) Overexpression of EGFR in head and neck squamous cell carcinoma is associated with inactivation of SH3GL2 and CDC25A genes. PLoS ONE 8(5):e63440
Mukherjee N, Dasgupta H, Bhattacharya R, Pal D, Roy R, Islam S, Alam N, Biswas J, Roy A, Roychoudhury S, Panda CK (2015) Frequent inactivation of MCC/CTNNBIP1 and overexpression of phospho-beta-catenin(Y654) are associated with breast carcinoma: clinical and prognostic significance. Biochim Biophys Acta 1862:1472–1484
Rigi-Ladiz MA, Kordi-Tamandani DM, Torkamanzehi A (2011) Analysis of hypermethylation and expression profiles of APC and ATM genes in patients with oral squamous cell carcinoma. Clin Epigenetics 3:6
Karbova E, Davidson B, Metodiev K, Tropé CG, Nesland JM (2002) Adenomatous polyposis coli (APC) protein expression in primary and metastatic serous ovarian carcinoma. Int J Surg Pathol 3:175–180
Song Y, Zhang C (2009) Hydralazine inhibits human cervical cancer cell growth in vitro in association with APC demethylation and re-expression. Cancer Chemother Pharmacol 63:605–613
Fang Jing-Yuan, Juan Lu, Chen Ying-Xuan, Yang Li (2003) Effects of DNA methylation on expression of tumor suppressor genes and proto-oncogene in human colon cancer cell lines. World J Gastroenterol 9:1976–1980
Dong Seung Myung, Kim Hy-Sook, Rha Seo-Hee, Sidransky D (1982) Promoter hypermethylation of multiple genes in carcinoma of the uterine cervix. Clin Cancer Res 7:1982–1986
Gao X, Zacharek A, Grignon D, Liu H, Sakr W, Porter A, Chen Y, Honn K (1995) High-frequency of loss of expression and allelic deletion of the apc and mcc genes in human prostate-cancer. Int J Oncol 6:111
Acknowledgements
The authors thank the Director of Chittaranjan National Cancer Institute, Kolkata, India. We are grateful to Professor (Dr.) H. zur Hausen and Professor (Dr. Mrs.) E.M. de Villiers for their generous gift of HPV-16/18 plasmids. This work was supported by CSIR (Council of Scientific and Industrial Research, Government of India)-JRF/NET grant [File No.09/030(0059)/2010-EMR-I] to Mr C.Chakraborty, grant [Sr. No. 2121130723] from UGC (University Grants Commission, Government of India) to Mr. Sudip Samadder, grant [SR/SO/HS-116/2007] from the Department of Science and Technology (DST), Government of India and grant [No. 60(0111)/14/EMR-II of dt.03/11/2014] from CSIR (Council of Scientific and Industrial Research, Government of India) to Dr. C. K. Panda.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11010_2017_3216_MOESM3_ESM.tif
Fig. S3 Kaplan–Meier 5-year survival probability curves with cumulative survival of CACX patients by Wnt3a protein expression. Supplementary material 3 (TIFF 1396 kb)
11010_2017_3216_MOESM7_ESM.docx
Table S4 Expression/methylation status of APC, β-catenin, p-β-catenin (Y654) and Wnt3a in different normal cervical epithelium. Supplementary material 7 (DOCX 33 kb)
11010_2017_3216_MOESM8_ESM.docx
Table S5 Relation between deletion (%) and methylation (%) of APC in CIN and CACX. Supplementary material 8 (DOCX 12 kb)
11010_2017_3216_MOESM10_ESM.docx
Table S7 Correlation with expression/methylation/deletion status of APC, β-catenin, p-β-catenin and Wnt3a in CIN and CACX. Supplementary material 10 (DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Chakraborty, C., Samadder, S., Roychowdhury, A. et al. Activation of Wnt-β-catenin pathway in basal–parabasal layers of normal cervical epithelium comparable during development of uterine cervical carcinoma. Mol Cell Biochem 443, 121–130 (2018). https://doi.org/10.1007/s11010-017-3216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-3216-5