Abstract
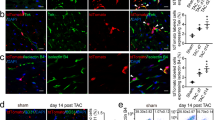
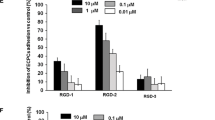
The aim of this study is to investigate the effect of evodiamine on fibroblast activation in cardiac fibroblasts and endothelial to mesenchymal transition (EndMT) in human umbilical vein endothelial cells (HUVECs). Neonatal rat cardiac fibroblasts were stimulated with transforming growth factor beta 1 (TGF-β1) to induce fibroblast activation. After co-cultured with evodiamine (5, 10 μM), the proliferation and pro-fibrotic proteins expression of cardiac fibroblasts were evaluated. HUVECs were also stimulated with TGF-β1 to induce EndMT and treated with evodiamine (5, 10 μM) at the same time. The EndMT response in the HUVECs was evaluated as well as the capacity of the transitioned endothelial cells migrating to surrounding tissue. As a result, Evodiamine-blunted TGF-β1 induced activation of cardiac fibroblast into myofibroblast as assessed by the decreased expressions of α-SMA. Furthermore, evodiamine reduced the increased protein expression of fibrosis markers in neonatal and adult rat cardiac fibroblasts induced by TGF-β1. HUVECs stimulated with TGF-β1 exhibited lower expression levels of CD31, CD34, and higher levels of α-SMA, vimentin than the control cells. This phenotype was eliminated in the HUVECs treated with both 5 and 10 μM evodiamine. Evodiamine significantly reduced the increase in migration ability that occurred in response to TGF-β1 in HUVECs. In addition, the activation of Smad2, Smad3, ERK1/2, and Akt, and the nuclear translocation of Smad4 in both cardiac fibroblasts and HUVEC were blocked by evodiamine treatment. Thus, evodiamine could prevent cardiac fibroblasts from activation into myofibroblast and protect HUVEC against EndMT. These effects may be mediated by inhibition of the TGFβ pathway in both cardiac fibroblasts and HUVECs.





Similar content being viewed by others
References
Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC (2016) Cardiac fibrosis: the fibroblast awakens. Circ Res 118:1021–1040. doi:10.1161/CIRCRESAHA.115.306565
A. L (2015) Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res 116:1269–1276
Fan Z and Guan J (2016) Antifibrotic therapies to control cardiac fibrosis. Biomater Res 20:13. doi:10.1186/s40824-016-0060-8
Lin F, Wang N, Zhang TC (2012) The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life 64:717–723. doi:10.1002/iub.1059
Krenning G, Zeisberg EM, Kalluri R (2010) The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 225:631–637. doi:10.1002/jcp.22322
Kanisicak O, Khalil H, Ivey MJ, Karch J, Maliken BD, Correll RN, Brody MJ, SC JL, Aronow BJ, Tallquist MD, Molkentin JD (2016) Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat Commun 7:12260. doi:10.1038/ncomms12260
Evrard SM LL, Michelis KC, Nomura-Kitabayashi A, Pandey G, Purushothaman KR, d’Escamard V, Li JR, Hadri L, Fujitani K, Moreno PR, Benard L, Rimmele P, Cohain A, Mecham B, Randolph GJ, Nabel EG, Hajjar R, Fuster V, Boehm M, Kovacic JC (2016) Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat Commun 7:11853
Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13:952–961. doi:10.1038/nm1613
Ge X CS, Liu M, Liang T, Liu C (2015) Evodiamine attenuates PDGF-BB-induced migration of rat vascular smooth muscle cells through activating PPARγ. Int J Mol Sci 16:28180–28193
Lv Q XY, Li G, Zou L, Zhang X, Ying M, Wang S, Guo L, Gao Y, Li G, Xu H, Liu S, Xie J, Liang S (2015) Beneficial effects of evodiamine on P2 × (4)-mediated inflammatory injury of human umbilical vein endothelial cells due to high glucose. Int Immunopharmacol 28:1044–1049
Chen TC, Chien CC, Wu MS, Chen YC (2016) Evodiamine from Evodia rutaecarpa induces apoptosis via activation of JNK and PERK in human ovarian cancer cells. Phytomedicine 23:68–78. doi:10.1016/j.phymed.2015.12.003
Liu LH XJ, Guo WW, Wu GY, Chen ZF, Yi JY, Zhang L, Zhang ZJ, Li Z (2014) Evodiamine activates AMPK and promotes adiponectin multimerization in 3T3-L1 adipocytes. J Asian Nat Prod Res 16:1074–1083
Zhao T ZX, Zhao Y, Zhang L, Bai X, Zhang J, Zhao X, Chen L, Wang L, Cui L (2014) Pretreatment by evodiamine is neuroprotective in cerebral ischemia: up-regulated pAkt, pGSK3β, down-regulated NF-κB expression, and ameliorated BBB permeability. Neurochem Res 39:1612–1620
Xue H CY, Wang X, Yue Y, Zhang W, Li X (2015) Rutaecarpine and evodiamine selected as β1-AR inhibitor candidates using β1-AR/CMC-offline-UPLC/MS prevent cardiac ischemia-reperfusion injury via energy modulation. J Pharm Biomed Anal 115:307–314
Wei J, Ching LC, Zhao JF, Shyue SK, Lee HF, Kou YR, Lee TS (2013) Essential role of transient receptor potential vanilloid type 1 in evodiamine-mediated protection against atherosclerosis. Acta Physiol (Oxf) 207:299–307. doi:10.1111/apha.12005
Fangfang Li YY Zhang N, Wu Q, Li J, Zhou M, Yang Z, Tang Q (2016) Evodiamine attenuates pressure overload-induced cardiac hypertrophy. Int J Clin Exp Med 9:in press.
Porter KE TN (2009) Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123:255–278
Li J, Zhang W, Jiao R, Yang Z, Yuan Y, Wu Q, Hu Z, Xiang S, Tang Q (2015) DIM attenuates TGF-beta1-induced myofibroblast differentiation in neonatal rat cardiac fibroblasts. Int J Clin Exp Pathol 8:5121–5128
Talman V, Ruskoaho H (2016) Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. doi:10.1007/s00441-016-2431-9
Peng X, Zhang Q, Zeng Y, Li J, Wang L, Ai P (2015) Evodiamine inhibits the migration and invasion of nasopharyngeal carcinoma cells in vitro via repressing MMP-2 expression. Cancer Chemother Pharmacol 76:1173–1184. doi:10.1007/s00280-015-2902-9
Zhou H, Chen X, Chen L, Zhou X, Zheng G, Zhang H, Huang W, Cai J (2014) Anti-fibrosis effect of scutellarin via inhibition of endothelial-mesenchymal transition on isoprenaline-induced myocardial fibrosis in rats. Molecules 19:15611–15623. doi:10.3390/molecules191015611
Lim H, Zhu YZ (2006) Role of transforming growth factor-beta in the progression of heart failure. Cell Mol Life Sci 63:2584–2596. doi:10.1007/s00018-006-6085-8
Yoshimatsu Y, Watabe T (2011) Roles of TGF-beta signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflam 2011:724080. doi:10.4061/2011/724080
Gunaratne A CE, El-Chabib TH, Carter D, Di Guglielmo GM (2015) aPKC alters the TGFβ response in NSCLC cells through both Smad-dependent and Smad-independent pathways. J Cell Sci 128:487–498
Meng ZJ, Wu N, Liu Y, Shu KJ, Zou X, Zhang RX, Pi CJ, He BC, Ke ZY, Chen L, Deng ZL, Yin LJ (2015) Evodiamine inhibits the proliferation of human osteosarcoma cells by blocking PI3K/Akt signaling. Oncol Rep 34:1388–1396. doi:10.3892/or.2015.4084
Author contributions
Qing–Qing Wu and Qi-Zhu Tang contributed to conception and designed experiments; Qing–Qing Wu and Yang Xiao, Xiao-Han Jiang and Yuan Yuan carried out experiments; Zheng Yang and Zhou-Yan Bian analyzed experimental results and revised the manuscript. Qing–Qing Wu and Wei Chang wrote and revised the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81470516, 81530012, and 81470402), Hubei Province’s Outstanding Medical Academic Leader program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wu, QQ., Xiao, Y., Jiang, XH. et al. Evodiamine attenuates TGF-β1-induced fibroblast activation and endothelial to mesenchymal transition. Mol Cell Biochem 430, 81–90 (2017). https://doi.org/10.1007/s11010-017-2956-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-017-2956-6