Abstract
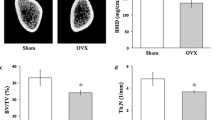
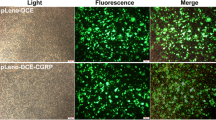
Osteoporosis, a systemic bone disorder, is prevalent in postmenopausal woman. Bone mesenchymal stem cells (BMSCs), precursors of osteogenic cells, may contribute to prevention or treatment of bone frustrate in osteoporosis. Recently, two studies suggested a role of calcitonin gene-related peptide (CGRP) in promoting osteogenesis of BMSCs under physiological conditions. However, the role of CGRP on BMSCs, which are derived from osteoporotic tissues, is unclear. Here, we investigated the role of CGRP on BMSCs isolated from female osteoporotic rats. Data showed that CGRP stimulated cell proliferation and inhibited cell apoptosis for short-term culture of BMSCs. Instead, CGRP induced BMSCs differentiation into the osteoblasts and promoted formation of calcified nodules after long-term culture. Moreover, CGRP gradually up-regulated expression levels of osteoporotic differentiation-related genes including alkaline phosphatase, Collagen type I, Bmp2, Osteonectin, and Runx2 during osteogenic differentiation. In conclusion, CGRP promoted proliferation and induced osteogenic differentiation and mineralization during female osteoporotic rat-derived BMSC differentiation. These findings support a potential role of CGRP on the prevention or treatment of osteoporotic fracture.







Similar content being viewed by others
References
Kanis JA, Melton LJ, Christiansen C, Johnston CC, Khaltaev N (1994) The diagnosis of osteoporosis. J Bone Miner Res 9:1137–1141. doi:10.1002/jbmr.5650090802
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936. doi:10.1016/S0140-6736(02)08761-5
Soen S (2014) Diagnostic criteria for primary osteoporosis: year 2012 revision. Clin Calcium 24:323–329. doi: CliCa1403323329
Pino AM, Rosen CJ, Rodriguez JP (2012) In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res 45:279–287. doi:10.4067/S0716-97602012000300009
Takada I, Kouzmenko AP, Kato S (2009) Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets 13:593–603. doi:10.1517/14728220902915310
Togari A, Arai M (2008) Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci 106:542–546
Serre CM, Farlay D, Delmas PD, Chenu C (1999) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25:623–629
Irie K, Hara-Irie F, Ozawa H, Yajima T (2002) Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech 58:85–90. doi:10.1002/jemt.10122
Imai S, Matsusue Y (2002) Neuronal regulation of bone metabolism and anabolism: calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc Res Tech 58:61–69. doi:10.1002/jemt.10119
Goto T, Nakao K, Gunjigake KK, Kido MA, Kobayashi S, Tanaka T (2007) Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides 41:25–31. doi:10.1016/j.npep.2006.11.002
Xu G, Jiang D (2014) The role and mechanism of exogenous calcitonin gene-related peptide on mesenchymal stem cell proliferation and osteogenetic formation. Cell Biochem Biophys 69:369–378. doi:10.1007/s12013-013-9809-z
Ma W, Chabot JG, Powell KJ, Jhamandas K, Dickerson IM, Quirion R (2003) Localization and modulation of calcitonin gene-related peptide-receptor component protein-immunoreactive cells in the rat central and peripheral nervous systems. Neuroscience 120:677–694
Lennerz JK, Ruhle V, Ceppa EP, Neuhuber WL, Bunnett NW, Grady EF, Messlinger K (2008) Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution. J Comp Neurol 507:1277–1299. doi:10.1002/cne.21607
Villa I, Dal Fiume C, Maestroni A, Rubinacci A, Ravasi F, Guidobono F (2003) Human osteoblast-like cell proliferation induced by calcitonin-related peptides involves PKC activity. Am J Physiol Endocrinol Metab 284:E627–E633. doi:10.1152/ajpendo.00307.2002
Lerner UH (2006) Deletions of genes encoding calcitonin/alpha-CGRP, amylin and calcitonin receptor have given new and unexpected insights into the function of calcitonin receptors and calcitonin receptor-like receptors in bone. J Musculoskelet Neuronal Interact 6:87–95
Wang L, Shi X, Zhao R, Halloran BP, Clark DJ, Jacobs CR, Kingery WS (2010) Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-kappaB activation, osteoclastogenesis and bone resorption. Bone 46:1369–1379. doi:10.1016/j.bone.2009.11.029
Li J, Wang Y, Li Y, Sun J, Zhao G (2014) The effect of combined regulation of the expression of peroxisome proliferator-activated receptor-gamma and calcitonin gene-related peptide on alcohol-induced adipogenic differentiation of bone marrow mesenchymal stem cells. Mol Cell Biochem 392:39–48. doi:10.1007/s11010-014-2016-4
Xie Z, Chen Y, Li Z, Bai G, Zhu Y, Yan R, Tan F, Chen YG, Guillemot F, Li L, Jing N (2011) Smad6 promotes neuronal differentiation in the intermediate zone of the dorsal neural tube by inhibition of the Wnt/beta-catenin pathway. Proc Natl Acad Sci U S A 108:12119–12124. doi:10.1073/pnas.1100160108
Jee WS, Yao W (2001) Overview: animal models of osteopenia and osteoporosis. J Musculoskelet Neuronal Interact 1:193–207
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191
Stevenson JC, Macdonald DW, Warren RC, Booker MW, Whitehead MI (1986) Increased concentration of circulating calcitonin gene related peptide during normal human pregnancy. Br Med J (Clin Res Ed) 293:1329–1330
Wimalawansa SJ (1996) Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr Rev 17:533–585. doi:10.1210/edrv-17-5-533
Mikami N, Miyagi Y, Sueda K, Takatsuji M, Fukada S, Yamamoto H, Tsujikawa K (2013) Calcitonin gene-related peptide and cyclic adenosine 5′-monophosphate/protein kinase A pathway promote IL-9 production in Th9 differentiation process. J Immunol 190:4046–4055. doi:10.4049/jimmunol.1203102
van Rossum D, Hanisch UK, Quirion R (1997) Neuroanatomical localization, pharmacological characterization and functions of CGRP, related peptides and their receptors. Neurosci Biobehav Rev 21:649–678
Hara-Irie F, Amizuka N, Ozawa H (1996) Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone 18:29–39
Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM (1983) Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature 304:129–135
Wang XY, Guo X, Cheng JC, Mi YL, Lai PY (2010) Involvement of calcitonin gene-related peptide innervation in the promoting effect of low-intensity pulsed ultrasound on spinal fusion without decortication. Spine (Phila Pa 1976) 35:E1539–E1545. doi:10.1097/BRS.0b013e3181cde89d
Mizuno M, Fujisawa R, Kuboki Y (2000) Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol 184:207–213. doi:10.1002/1097-4652(200008)184:2<207:AID-JCP8>3.0.CO;2-U
Gruber R, Mayer C, Schulz W, Graninger W, Peterlik M, Watzek G, Luyten FP, Erlacher L (2000) Stimulatory effects of cartilage-derived morphogenetic proteins 1 and 2 on osteogenic differentiation of bone marrow stromal cells. Cytokine 12:1630–1638. doi:10.1006/cyto.2000.0760
Huang W, Carlsen B, Wulur I, Rudkin G, Ishida K, Wu B, Yamaguchi DT, Miller TA (2004) BMP-2 exerts differential effects on differentiation of rabbit bone marrow stromal cells grown in two-dimensional and three-dimensional systems and is required for in vitro bone formation in a PLGA scaffold. Exp Cell Res 299:325–334. doi:10.1016/j.yexcr.2004.04.051
Wang L, Huang Y, Pan K, Jiang X, Liu C (2010) Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng 38:77–87. doi:10.1007/s10439-009-9841-8
Tian G, Zhang G, Tan YH (2013) Calcitonin gene-related peptide stimulates BMP-2 expression and the differentiation of human osteoblast-like cells in vitro. Acta Pharmacol Sin 34:1467–1474. doi:10.1038/aps.2013.41
Rosen V (2009) BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev 20:475–480. doi:10.1016/j.cytogfr.2009.10.018
James AW (2013) Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica (Cairo) 2013:684736. doi:10.1155/2013/684736
Shakibaei M, Shayan P, Busch F, Aldinger C, Buhrmann C, Lueders C, Mobasheri A (2012) Resveratrol mediated modulation of Sirt-1/Runx2 promotes osteogenic differentiation of mesenchymal stem cells: potential role of Runx2 deacetylation. PLoS ONE 7:e35712. doi:10.1371/journal.pone.0035712
Acknowledgments
We thank all other members in the laboratory for their technical supports and helpful discussions. This study is supported by National Natural Science Foundation of China (No: 81260273).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Liang and Xianglong Zhuo contributed equally to this study.
Rights and permissions
About this article
Cite this article
Liang, W., Zhuo, X., Tang, Z. et al. Calcitonin gene-related peptide stimulates proliferation and osteogenic differentiation of osteoporotic rat-derived bone mesenchymal stem cells. Mol Cell Biochem 402, 101–110 (2015). https://doi.org/10.1007/s11010-014-2318-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-014-2318-6