Abstract
Objectives
With a fourth of all under-five children affected, stunting remains one of the biggest health challenges worldwide. Even though the main underlying factors are known, the exact pathways to stunting varying in affected regions, and interventions thus need to be tailored to the local contexts. This study aimed assessing and comparing factors associated with stunting in two understudied sub-Saharan urban contexts with some of the highest stunting prevalence globally: Bangui, Central African Republic (~ 36%) and Antananarivo, Madagascar (42%).
Methods
We performed a case–control study on 175 + 194 stunted and 237 + 230 non-stunted control children aged 2–5 years and matched for age, gender and district of residency. Factors associated with stunting were identified using a standardized, paper questionnaire delivered by trained interviewers. Statistical analysis was done using logistic regression modelling.
Results
In both sites, formal maternal education lowered the risk of being stunted and restricted access to soap, suffering of anaemia and low birth weight were associated with higher risk of stunting. Short maternal stature, household head different from parents, diarrhoea and coughing were associated with an increased risk and continuing breastfeeding was associated with a lower risk of stunting in Antananarivo. Previous severe undernutrition and dermatitis/ fungal skin infections were associated with higher and changes in diet during pregnancy with lower risk of stunting in Bangui.
Conclusions
Our results suggest maternal education, antenatal care, iron supplementation and simple WASH interventions such as using soap and infection control as general and breastfeeding (Antananarivo) or better nutrition (Bangui) as area-specified interventions.
Similar content being viewed by others
Significance
Although global trends reveal a decline in stunted child growth, stunting still affects one out of three children in sub-Saharan Africa. Madagascar and the Central African Republic are among the most affected countries globally. Yet we lack data on associated factors in these countries. This article describes nutritional, family, household and socioeconomic factors associated with stunting in the two capital cities, Antananarivo and Bangui. This is the first study specifically assessing stunting-associated factors in these two geographical regions providing a locally adapted roadmap for the implementation of powerful intervention and prevention strategies in these two settings.
Introduction
Stunted child growth reflects underlying chronic undernutrition and is of major global public health concern. Stunting is characterized by a height of more than two standard deviations below the World Health Organization (WHO) age-specific growth reference standard (Group, 2007). Stunted children have a threefold higher mortality compared to non-stunted children (McDonald et al., 2013; Victora et al., 2021). Children stunted in early life also are at higher risk of displaying cognitive impairments later in life and of experiencing metabolic disease (Prendergast & Humphrey, 2014). Sub-Saharan Africa is one of the most affected regions for child stunting (Local Burden of Disease Child Growth Failure, 2020). The Central African Republic (CAR) and Madagascar, where this study was conducted, are two of the world’s most affected countries, with 42% of Malagasy and 40% of CAR children experiencing stunted growth. This syndrome is associated with several aetiologies, notably a poor, unbalanced diet, insufficient vitamin/micronutrient intake, repeated infections and changes to the gastrointestinal system, especially chronic inflammation and changes to the microbiota (Prendergast & Humphrey, 2014; Vonaesch et al., 2018a). Stunted child growth is also influenced by socio-economic and political factors, including familial resources and configuration, access to health care, and the broader political economic conditions in which children and their families live (Victora et al., 2021). Factors associated with stunting are varying in each context, even if seemingly similar. Indeed, a meta-analysis in 19 countries showed that interventions worked best when country, community and program context were taken into account (Hossain et al., 2017). It is therefore important to know which factors are present in which context in order to tailor interventions to the local need.
Globally, major efforts have been made to decrease stunting prevalence and nutrition-specific and nutrition-sensitive interventions are included in almost all national health plans (Victora et al., 2021). Although the WHO Global Nutrition Targets aim to reduce stunting by 40% by 2025, the CAR and Madagascar have only achieved limited progress in reaching Global Nutrition Targets (Local Burden of Disease Child Growth Failure, 2020).
Because stunting is inherently multifactorial, no single intervention can tackle childhood undernutrition and stunting (Argaw et al., 2019). Rather, this syndrome requires multi-sectorial, coordinated nutrition-sensitive and nutrition-specific interventions that address all conditions facilitating chronic childhood undernutrition (Heidkamp et al., 2021). As associated factors are context-specific, local studies providing in-depth understanding of factors contributing to stunting are crucial to identify successful intervention strategies for a given context (Carter et al., 2020).
The objective of this study was to assess and compare factors associated with stunted child growth in two sub-Saharan African cities having among the highest prevalence of stunted child growth worldwide: Bangui, the capital of the CAR and Antananarivo, the capital of Madagascar.
Methods
Study Design and Study Population
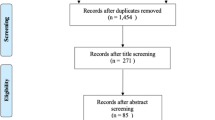
The AFRIBIOTA study (Vonaesch et al., 2018b) is a case–control study for stunting in children aged 2–5 years in Bangui, CAR and Antananarivo, Madagascar. The choice of the two study sites was based on the high stunting prevalence and the fact that the consortium worked previously together on a project on diarrhoeal diseases (Breurec et al., 2016; Randremanana et al., 2016). Inclusion criteria were HIV-negative children, neither suffering from acute malnutrition nor from any other severe disease, living in the 6st, 7st or 8th district of Bangui or in two neighbourhoods of Antananarivo (Ankasina or Andranomanalina Isotry). Included children were admitted to the hospital for sample collection and anthropometric measurement. Assuming an alpha-error of 0.05%, a power of 80% and an expected 20% exposure in the cases, we needed at least 169 children per group, hence 676 individuals in the two countries. The final sample size analysed in this study was of 836 children (Fig. 1). Stunted and control children were matched according to age in years, gender, neighbourhood and season of inclusion (dry or wet season). Recruitment took place between December 2016 and March 2018 in Antananarivo and between January 2017 and May 2018 in Bangui. Detailed recruitment procedures are given in the Supplementary methods and the study protocol (Vonaesch et al., 2018b).
Anthropometric Measurements
Height was measured by trained personnel to the nearest 0.1 cm in a standing position using collapsible height boards (Antananarivo: ShorrBoard Measuring Board, Maryland, USA; Bangui: height board provided by UNICEF); weight was measured to the nearest 100 g using a weighing scale (Antananarivo: KERN, ref. MGB 150K100 and EKS, Inter-équipement Madagascar; Bangui: weighting scale provided by UNICEF) and mid-upper arm circumference (MUAC) was measured using commercial MUAC tape (provided by UNICEF) to the nearest 0.1 cm. Cut-offs were based on the official cut-offs defined by WHO (Onis, 2006).
Data Collection
A standardized, paper questionnaire in French that was translated ad hoc to the local languages Sangho and Malagasy was used in both study sites and data was entered in double in an Access database. The questionnaire included information about children’s age, gender, family structure, socioeconomic status indicators, sanitary indicators, data about the mother’s pregnancy and child’s and family nutrition and feeding practices. A wealth index was created based on the minimal set of assets, leading to a separation of subjects in three distinct groups in a principal component analysis (PCoA). Details of the wealth index are given in the extended methods. Each child was further examined for comorbidities and venous blood was collected. Complete blood count, C-reactive protein (CRP) and ferritin levels were measured. Ferritin levels were corrected for systemic inflammation (Thurnham et al., 2010), haemoglobin values were adjusted for altitude (Centers for Disease, 1989; Sullivan et al., 2008) and anaemia was defined as less than 110 g/l, according to WHO criteria (OMS, 2011; Onis, 2006). A dietary diversity score (DDS) was calculated based on a 24 h recall (World Health Organization, 2007a, 2007b). Mother’s nutritional status was based on the Body Mass Index (BMI). Non-pregnant mothers were classified by BMI categories as defined by the WHO and pregnant mothers according to the categories proposed by Ververs (Ververs et al., 2013). Clinical parameters such as cough (observed and reported by the mothers), dermatitis (as visible dermal affections of various origins diagnosed by a medical doctor), diarrhoea (> 3 loose stools/day), and tooth decay were assessed during a clinical examination. Previous episode of severe acute malnutrition and perceived low birth weight was based on mother’s recall.
Statistical Analysis
The statistical analysis was performed with Stata 13. Significance level was fixed for all analyses at 0.05 and all tests were performed bilaterally. Categorical variables were expressed as percentages; quantitative variables were expressed as a mean (± Standard Deviation) or median (interquartile range). The stunted vs. non-stunted groups were compared using Chi2 or Fisher Exact test for qualitative variables and the Student t test or the Mann–Whitney U test for quantitative variables. All variables were assessed in a bivariate analysis. Factors associated with stunting in bivariate analysis with a P value of < 0.2 were checked for potential confounding factors and interactions and then included in a backward logistic regression. As we did not get a perfect matching for age, gender and season of inclusion, these variables were forced in the multivariate model. Results are reported as adjusted OR with 95% CI, corrected for age in years, gender, season of inclusion and country of origin.
Results
General Characteristics of Study Participants and Their Mothers
The final dataset analysed comprised 409 children from Bangui and 424 children from Antananarivo (Fig. 1; Table 1). The general characteristics of the study population are summarized in Table 1. In both study sites, most children lived with their mothers (Bangui: 93.0%, Antananarivo: 97.0%, P = 0.01) and with their fathers (Bangui: 75.0%; Antananarivo: 82.0%, P = 0.02). Bangui households tended to have a higher socioeconomic score (11.0% in lowest, 81.9% in middle and 7.1% in highest income group) compared to Madagascar (66.0% in lowest, 12.2% in middle and 3.8% in highest income group, P < 0.001). In both countries, most families had either the father or the mother working. Nevertheless, 10.6% of Antananarivo children and 22.5% of Bangui children lived in a household in which someone other than the father or mother was the primary earner (P < 0.001). Mothers’ characteristics are summarized in Table S1.
Nutritional Habits and Young Child Feeding
Roughly a quarter of mothers in Bangui and 5.0% in Madagascar changed their nutrition during pregnancy. The most commonly reported changes were more fruits, vegetables, meat and fish in Antananarivo, and increased consumption of dishes like “Dadawa” (fermented beans that season dishes and are understood to be highly nutritious in central and west Africa), “Kôkô” (Gnetum species, which grow in forest tangle and serve as the basis for leaf sauces), “Goussa” (squash seeds, pounded and used in sauces), “Nguiriki” (sauce) and other dishes rich in beans, peanuts, palm oil, meat, fish, caterpillars and green leafy vegetables in Bangui. Mothers claimed that these foods were “highly nutritious”.
Mothers or caregivers introduced complementary food to children earlier [median of 3 (IQR 1–10) vs. 6 (IQR: 1–8) months; Pr < 0.0001] and breastfed for a shorter period [17.3 (IQR: 16–19) vs. 24.6 (IQR: 16–19) months on average; Pr < 0.0001] in Bangui than in Antananarivo. (Fig. 2, Table 2). Eighty percent (80.0%) of all Bangui children and 90.0% of Antananarivo children were at least partially breastfed throughout their first year of life.
Breastfeeding characteristics in the two study sites. Kernel density plots of A the age of first introduction of complementary food and B the age of weaning as reported by the mother/primary caregiver. Data from Antananarivo, Madagascar is indicated in blue, data from the Bangui, Central African Republic (CAR) in red
In Antananarivo, the most common complementary food items reported by caregivers was “Koba aina” (enriched ready-to-cook product consisting of corn, rice and peanuts), combined in some households with certain vegetables (potatoes, carrot) or commercially available wheat- and milk-based children’s powder (“Farilac”), milk, biscuits or cheese. Only 11/424 (2.5%) of caregivers reported having regularly given their infant meat and 2/424 (0.5%) fish during the weaning period. In Bangui, the majority of caregivers gave their children a maize and water-based runny porridge (“Koulou”) and less frequently a rice porridge, commercial wheat- and milk-based children’s powder (“phosphatine”, “Bledina”) or very rarely a plantain- or millet-based porridge. In total, 38/409 (9.0%) reported combining this base on a routine basis with vegetables (green leafy vegetables and/or tomatoes), 19/409 (4.5%) with fish, 10/409 (2.5%) with meat, 7/409 (1.5%) with peanuts, 2/409 (0.5%) with eggs, 2/409 (0.5%) with legumes and 1/409 (0.3%) with caterpillars. In both countries, nutritional diversity was on average below the recommended food diversity score of 4 (Table 2) yet 64% of families in Antananarivo and 54% Bangui reporting having given their children at least four different food groups the previous day. Fifty three percent (53%) of families in Antananarivo and 41% of families in Bangui reported having provided their children with meat, and 24% and 45%, respectively, offered fish in the previous day.
Based on anthropological observations in the communities and in accordance with other studies performed in Madagascar (Rakotomanana et al., 2017a, 2017b; Rakotonirainy et al., 2018) we suspect meat and fish quantities to be minimal, and diet in both countries to be primarily starch-based.
Sanitary Conditions
Sanitary conditions in both settings were challenging for families and are summarized in Table S2. In Bangui, only 16.0% of the households had constant access to soap, compared to 45.0% in Antananarivo. Thirty four percent (34%) of all households in Bangui and 56% in Antananarivo reported to be exposed to a possible contamination source (open toilet, trash bin, wastewater evacuation canal) with a distance of less than 5 m to their house. In 5% of Antananarivo households and 21% of all Bangui households, poultry lived with the family in the same dwelling.
Prevalence of Micronutrient Deficiencies and Other Co-morbidities
In both countries, children displayed several comorbidities (Table S3). Anaemia was very widespread, with 47% of Bangui children and 20% of Antananarivo children showing haemoglobin levels below 11 g/l. Urinary iodine levels were only measured in Antananarivo. The median level was 87 ug/l (IQR 35; 170), showing moderate iodine deficiency compared to WHO references values for school age children (World Health Organization, 2007a, 2007b). Other observed comorbidities on the day of inclusion included mild respiratory infections, visible caries (38% in Antananarivo), dermatitis and diarrhoea. Virtually all Bangui children (96%) and roughly a quarter (28%) of Antananarivo children did not have full vaccine coverage.
Factors Associated with Stunted Growth
In Antananarivo, social factors such as mother’s height, mother’s education and a head of household other than the parents were significantly associated with stunted child growth (Table 3). Comorbidities at the time of inclusion, including cough (aOR1.73; CI 1.04; 2.88), diarrhoea (aOR 15.15; CI 1.48; 154.58) and anaemia (aOR 3.60; CI 1.88; 6.88) were significantly associated with stunted child growth, as were early life factors such as perceived birth weight (aOR 2.23; CI 1.20; 4.16 of low vs. normal and aOR 0.30; CI 0.17; 0.54 of high vs. normal) or prolonged breastfeeding (aOR 0.30, CI 0.13; 0.70 of at least one year breastfeeding vs. less). Not having constant access to soap was associated with an 80% increase in stunted child growth.
In Bangui, the main factors associated with stunted child growth were maternal education (85% fewer stunted children born to mothers who completed high school compared to no formal education) and different biological factors, such as anaemia (aOR 2.2; CI 1.27; 3.82), low birth weight (aOR 2.81; CI 1.13; 6.98 for small vs. normal weight children), previous episode of severe acute undernutrition (aOR 5.52; CI 1.9; 16.07) and dermatitis at time of inclusion (aOR 4.32; CI 2.08; 8.99). Not having constant access to soap was associated with more than threefold increase in stunted child growth (aOR 3.38; CI 1.68; 6.80). The results of the multivariate analysis of factors associated with stunting in Bangui are summarized in Table 4 and from the two countries pooled in Table S4.
Discussion
Even though Madagascar and the CAR are among the most affected countries globally, we have little data related to factors associated with stunting in these two settings. Our study contributes therefore important data on stunting-associated factors, which can serve as baseline for targeted interventions by policy makers.
In our study low birth weight, lack of access to soap as well as comorbidities such as anaemia, diarrhoea, dermatitis or respiratory disease are risk factors and higher education of the mother is a protective factor for stunting in both study settings, reflecting the most common stunting-associated factors globally (Hawkes et al., 2020).
Roughly a fifth of Antananarivo children and half of Bangui children included had haemoglobin levels indicative of anaemia. We observed similar levels of low iron in Antananarivo (21%), but only a small proportion of children in Bangui had low iron levels (9%). This discrepancy between iron levels and anaemia warrants further investigation. Our urinary iodine measurements in Antananarivo align with a previous report, which showed moderate iodine deficiency across the country. Madagascar is currently reviving a salt-based iodine supplementation program (Randremanana et al., 2019). We did not find any association between food diversity and stunting in the two study sites. These observations clearly necessitate more thorough mixed-methods studies that assess consumed food items and their quantification.
In a study in 137 low- and middle-income countries (LMIC), poor sanitation has been identified to be worldwide the second largest burden to child stunting after low birth weight (Danaei et al., 2016). In contrast to this observation are three recent, very well designed and large-scale randomized clinical trials assessing the benefit of water, sanitation and hygiene (WASH) interventions on child stunting. None of these trials found an effect of WASH on stunting (Cumming et al., 2019; Humphrey et al., 2019; Prendergast et al., 2019). Researchers of these studies acknowledge that interventions must target local exposures, enteric disease burden and cofactors, an approach they termed “transformative WASH”. In accordance, in a recent systematic review assessing fourteen programs to reduce stunting in 19 LMIC, the authors found that similar interventions led to different outcomes in different contexts (Hossain et al., 2017). In our study, of all of the sanitation variables tested, only the presence of soap protected against stunting. This suggests that hand hygiene might be the easiest and most effective WASH intervention to be implemented in Antananarivo and Bangui.
Animals, especially if reared in the same physical space as children, have previously been reported to be associated with stunted child growth (Monira et al., 2020); although these associations vary across different settings (Headey et al., 2017). In our study, roughly a third of all Antananarivo households and more than two-thirds of Bangui households owned poultry. In Antananarivo, owning poultry was associated with a roughly two-fold higher risk of stunting in the multivariate model. No association between poultry and stunting was observed in Bangui. This discrepancy between the two study sites could be due to the fact that Antananarivo is much more densely populated than Bangui, leaving animals in closer proximity to their owners and their children. Indeed, faecal material from chicken and pigs can potentially lead to zoonotic diseases, such as salmonellosis, cryptosporidiosis, pathogenic E. coli infections, giardiasis and Campylobacter infections, all of which have been associated with stunted child growth (Rogawski et al., 2017; Sanchez et al., 2020). Our data assessing the microbiome of children in the same setting shows that faecal asymptomatic carriage of pathobionts (especially the genera Shigella/Escherichia and Campylobacter) is associated with stunted child growth (Vonaesch et al., 2018a). Targeted studies on asymptomatic pathogen carriage and their relation with child stunting are currently ongoing.
Beside shared factors, the two study sites also displayed specific factors associated with stunting (Fig. 3).
In Antananarivo, short maternal stature, household head different from parents, diarrhoea and coughing were associated with an increased risk and continuing breastfeeding was associated with a lower risk of stunting. This suggests that in Antananarivo, beside anaemia and low birth weight, infections are one of the main pathways leading to stunted child growth.
Further, the highest socio-economic group seemed to have the highest odds of being stunted. A possible explanation to this puzzling result might be that the recruitment was taking place in two of the poorest neighborhoods in Antananarivo, hence stratifying the poor population to three categories (the highest category representing the richest amongst the poor) rather than assessing for families, which indeed do have a higher socioeconomic score. It is further possible that this socioeconomic group has specific behavioral patterns that are favoring stunting that have not been captured through our data collection. Anthropological studies are ongoing in both study sites.
Previous studies outside the capital city found child gender and age (McCuskee et al., 2018; Rakotomanana et al., 2017a, 2017b; Remonja et al., 2017), low maternal stature (McCuskee et al., 2018; Rakotomanana et al., 2017a, 2017b) and birth weight (McCuskee et al., 2018; Remonja et al., 2017), inadequately spaced birth (Remonja et al., 2017), low paternal height (McCuskee et al., 2018), socioeconomic score (Remonja et al., 2017), infection with the helminth Trichuris trichiura (Remonja et al., 2017) and food diversity score (Aiga et al., 2019), to be associated with stunted child growth. This suggests that the pathways leading to stunting in the capital city are slightly different than in other parts of the country, calling for context-specific interventions.
In Bangui, previous severe undernutrition and dermatitis/fungal skin infections were associated with higher and changes in diet during pregnancy with lower risk of stunting. Together with the very high prevalence of anaemia and the low levels of breastfeeding, stunted child growth in Bangui may be primarily related to nutritional deficits, both, in pregnant mothers as well as their infants, thus suggesting the implementation of nutrition-specific interventions.
The main strength of our study lies in the extensive cohort with almost 1000 children and its rigorous control using standardized questionnaires, enabling robust statistical analysis. Our study has however also some weaknesses: the choice to include only children between 2 and 5 years could lead to missing important factors associated with early life stunting. Further, information regarding the mother’s pregnancy, breastfeeding and early complementary feeding may be subject to recall bias. Also, due to the matched design, we could not assess for the effect of gender and we did not assess for birth spacing and paternal height, zinc or vitamin A status which are factors associated with stunting in previous studies.
In conclusion, our results suggest maternal education, antenatal care, iron supplementation and simple WASH interventions such access to soap and infection control and breastfeeding (Antananarivo) or better nutrition (Bangui) as specific interventions in the two study settings. Our results are an important milestone for these countries, as they provide a locally adapted roadmap for the implementation of targeted intervention and prevention strategies against childhood stunting.
References
Aiga, H., Abe, K., Andrianome, V. N., Randriamampionona, E., Razafinombana, A. R., Murai, T., & Hara, M. (2019). Risk factors for malnutrition among school-aged children: A cross-sectional study in rural Madagascar. BMC Public Health, 19(1), 773.
Argaw, A., Hanley-Cook, G., De Cock, N., Kolsteren, P., Huybregts, L., & Lachat, C. (2019). Drivers of under-five stunting trend in 14 low- and middle-income countries since the turn of the millennium: A multilevel pooled analysis of 50 demographic and health surveys. Nutrients. https://doi.org/10.3390/nu11102485
Breurec, S., Vanel, N., Bata, P., Chartier, L., Farra, A., Favennec, L., Franck, T., Giles-Vernick, T., Gody, J.-C., Luong Nguyen, L. B., Onambélé, M., Rafaï, C., Razakandrainibe, R., Tondeur, L., Tricou, V., Sansonetti, P., & Vray, M. (2016). Etiology and epidemiology of diarrhea in hospitalized children from low income country: A matched case-control study in Central African Republic. PLoS Neglected Tropical Diseases, 10(1), e0004283.
Carter, A., Akseer, N., Ho, K., Rothschild, O., Bose, N., Binagwaho, A., Hirschhorn, L. R., Price, M., Muther, K., Panjabi, R., Freeman, M. C., Bednarczyk, R. A., & Bhutta, Z. A. (2020). A framework for identifying and learning from countries that demonstrated exemplary performance in improving health outcomes and systems. BMJ Global Health. https://doi.org/10.1136/bmjgh-2020-002938
Centers for Disease Control. (1989). CDC criteria for anemia in children and childbearing-aged women. MMWR Morbidity and Mortality Weekly Report, 38(22), 400–404.
Cumming, O., Arnold, B. F., Ban, R., Clasen, T., Esteves Mills, J., Freeman, M. C., Gordon, B., Guiteras, R., Howard, G., Hunter, P. R., Johnston, R. B., Pickering, A. J., Prendergast, A. J., Pruss-Ustun, A., Rosenboom, J. W., Spears, D., Sundberg, S., Wolf, J., Null, C., … Colford, J. M., Jr. (2019). The implications of three major new trials for the effect of water, sanitation and hygiene on childhood diarrhea and stunting: A consensus statement. BMC Medicine, 17(1), 173.
Danaei, G., Andrews, K. G., Sudfeld, C. R., Fink, G., McCoy, D. C., Peet, E., Sania, A., Smith Fawzi, M. C., Ezzati, M., & Fawzi, W. W. (2016). Risk factors for childhood stunting in 137 developing countries: A comparative risk assessment analysis at global, regional, and country levels. PLoS Medicine, 13(11), e1002164.
Hawkes, C., Ruel, M. T., Salm, L., Sinclair, B., & Branca, F. (2020). Double-duty actions: Seizing programme and policy opportunities to address malnutrition in all its forms. Lancet, 395(10218), 142–155.
Headey, D., Nguyen, P., Kim, S., Rawat, R., Ruel, M., & Menon, P. (2017). Is exposure to animal feces harmful to child nutrition and health outcomes? A multicountry observational analysis. American Journal of Tropical Medicine and Hygiene, 96(4), 961–969.
Heidkamp, R. A., Piwoz, E., Gillespie, S., Keats, E. C., D’Alimonte, M. R., Menon, P., Das, J. K., Flory, A., Clift, J. W., Ruel, M. T., Vosti, S., Akuoku, J. K., & Bhutta, Z. A. (2021). Mobilising evidence, data, and resources to achieve global maternal and child undernutrition targets and the sustainable development goals: An agenda for action. Lancet, 397(10282), 1400–1418.
Hossain, M., Choudhury, N., Adib Binte Abdullah, K., Mondal, P., Jackson, A. A., Walson, J., & Ahmed, T. (2017). Evidence-based approaches to childhood stunting in low and middle income countries: a systematic review. Archives of Disease in Childhood, 102(10), 903–909.
Humphrey, J. H., Mbuya, M. N. N., Ntozini, R., Moulton, L. H., Stoltzfus, R. J., Tavengwa, N. V., Mutasa, K., Majo, F., Mutasa, B., Mangwadu, G., Chasokela, C. M., Chigumira, A., Chasekwa, B., Smith, L. E., Tielsch, J. M., Jones, A. D., Manges, A. R., Maluccio, J. A., Prendergast, A. J., & SHINE Trial Team. (2019). Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: A cluster-randomised trial. The Lancet. Global Health, 7(1), e132–e147.
Local Burden of Disease Child Growth Failure Collaborators. (2020). Mapping child growth failure across low- and middle-income countries. Nature, 577(7789), 231–234.
McCuskee, S., Garchitorena, A., Miller, A. C., Hall, L., Ouenzar, M. A., Rabeza, V. R., Ramananjato, R. H., Razanadrakato, H. R., Randriamanambintsoa, M., Barry, M., & Bonds, M. H. (2018). Child malnutrition in Ifanadiana district, Madagascar: Associated factors and timing of growth faltering ahead of a health system strengthening intervention. Global Health Action, 11(1), 1452357.
McDonald, C. M., Olofin, I., Flaxman, S., Fawzi, W. W., Spiegelman, D., Caulfield, L. E., Black, R. E., Ezzati, M., Danaei, G., & Nutrition Impact Model Study. (2013). The effect of multiple anthropometric deficits on child mortality: meta-analysis of individual data in 10 prospective studies from developing countries. American Journal of Clinical Nutrition, 97(4), 896–901.
Monira, S., Islam Bhuyian, M. S., Parvin, T., Uddin, I. M., Zohura, F., Hasan, M. T., Biswas, S. K., Hasan, K., Masud, J., Rashid, M. U., Rahman, Z., Papri, N., Rafique, R., Islam, A., Barman, I., Tuz Jubyda, F., Johura, F. T., Sultana, M., Sanin, K. I., … George, C. M. (2020). Child mouthing of soil and presence of animals in child sleeping spaces are associated with growth faltering among young children in Urban Dhaka, Bangladesh (CHoBI7 program). Tropical Medicine & International Health. https://doi.org/10.1111/tmi.13417
OMS. (2011). Concentrations en hémoglobine permettant de diagnostiquer l’anémie et d’en évaluer la sévérité. Retrieved May 09, 2019
Onis, M. (2006). WHO child growth standards based on length/height, weight and age. Acta Paediatrica, 95(S450), 76–85.
Prendergast, A. J., Chasekwa, B., Evans, C., Mutasa, K., Mbuya, M. N. N., Stoltzfus, R. J., Smith, L. E., Majo, F. D., Tavengwa, N. V., Mutasa, B., Mangwadu, G. T., Chasokela, C. M., Chigumira, A., Moulton, L. H., Ntozini, R., Humphrey, J. H., & SHINE Trial Team. (2019). Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on stunting and anaemia among HIV-exposed children in rural Zimbabwe: A cluster-randomised controlled trial. The Lancet Child & Adolescent Health, 3(2), 77–90.
Prendergast, A. J., & Humphrey, J. H. (2014). The stunting syndrome in developing countries. Paediatrics and International Child Health, 34(4), 250–265.
Rakotomanana, H., Gates, G. E., Hildebrand, D., & Stoecker, B. J. (2017a). Determinants of stunting in children under 5 years in Madagascar. Maternal & Child Nutrition. https://doi.org/10.1111/mcn.12409
Rakotomanana, H., Gates, G. E., Hildebrand, D., & Stoecker, B. J. (2017b). Situation and determinants of the infant and young child feeding (IYCF) indicators in Madagascar: Analysis of the 2009 demographic and health survey. BMC Public Health, 17(1), 812.
Rakotonirainy, N. H., Razafindratovo, V., Remonja, C. R., Rasoloarijaona, R., Piola, P., Raharintsoa, C., & Randremanana, R. V. (2018). Dietary diversity of 6- to 59-month-old children in rural areas of Moramanga and Morondava districts, Madagascar. PLoS ONE, 13(7), e0200235.
Randremanana, R. V., Bastaraud, A., Rabarijaona, L. P., Piola, P., Rakotonirina, D., Razafinimanana, J. O., Ramangakoto, M. H., Andriantsarafara, L., Randriamasiarijaona, H., Tucker-Brown, A., Harimanana, A., & Namana, S. (2019). First national iodine survey in Madagascar demonstrates iodine deficiency. Maternal & Child Nutrition, 15(2), e12717.
Randremanana, R. V., Razafindratsimandresy, R., Andriatahina, T., Randriamanantena, A., Ravelomanana, L., Randrianirina, F., & Richard, V. (2016). Etiologies, risk factors and impact of severe diarrhea in the under-fives in Moramanga and Antananarivo, Madagascar. PLoS ONE, 11(7), e0158862.
Remonja, C. R., Rakotoarison, R., Rakotonirainy, N. H., Mangahasimbola, R. T., Randrianarisoa, A. B., Jambou, R., Vigan-Womas, I., Piola, P., & Randremanana, R. V. (2017). The importance of public health, poverty reduction programs and women’s empowerment in the reduction of child stunting in rural areas of Moramanga and Morondava, Madagascar. PLoS ONE, 12(10), e0186493.
Rogawski, E. T., Guerrant, R. L., Havt, A., Lima, I. F. N., Medeiros, P. H. Q. S., Seidman, J. C., McCormick, B. J. J., Babji, S., Hariraju, D., Bodhidatta, L., Shrestha, J., Anania, J., Maro, A., Samie, A., Yori, P. P., Qureshi, S., Mahfuz, M., Bessong, P. O., Kosek, M. N., … Investigators, M.-E.N. (2017). Epidemiology of enteroaggregative Escherichia coli infections and associated outcomes in the MAL-ED birth cohort. PLoS Neglected Tropical Diseases, 11(7), e0005798.
Sanchez, J. J., Alam, A., Stride, C. B., Haque, A., Das, S., Mahfuz, M., Roth, D. E., Sly, P. D., Long, K. Z., & Ahmed, T. (2020). Campylobacter infection and household factors are associated with childhood growth in urban Bangladesh: An analysis of the MAL-ED study. PLoS Neglected Tropical Diseases, 14(5), e0008328.
Sullivan, K. M., Mei, Z., Grummer-Strawn, L., & Parvanta, I. (2008). Haemoglobin adjustments to define anaemia. Tropical Medicine & International Health, 13(10), 1267–1271.
Thurnham, D. I., McCabe, L. D., Haldar, S., Wieringa, F. T., Northrop-Clewes, C. A., & McCabe, G. P. (2010). Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta-analysis. American Journal of Clinical Nutrition, 92(3), 546–555.
Ververs, M. T., Antierens, A., Sackl, A., Staderini, N., & Captier, V. (2013). Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Currents. https://doi.org/10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8
Victora, C. G., Christian, P., Vidaletti, L. P., Gatica-Dominguez, G., Menon, P., & Black, R. E. (2021). Revisiting maternal and child undernutrition in low-income and middle-income countries: Variable progress towards an unfinished agenda. Lancet, 397(10282), 1388–1399.
Vonaesch, P., Morien, E., Andrianonimiadana, L., Sanke, H., Mbecko, J.-R., Huus, K. E., Naharimanananirina, T., Gondje, B. P., Nigatoloum, S. N., Vondo, S. S., Kaleb Kandou, J. E., Randremanana, R., Rakotondrainipiana, M., Mazel, F., Djorie, S. G., Gody, J.-C., Finlay, B. B., Rubbo, P.-A., Wegener-Parfrey, L., … Sansonetti, P. J. (2018a). Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proceedings of the National Academy of Sciences of the United States of America, 115(36), E8489–E8498.
Vonaesch, P., Randremanana, R., Gody, J. C., Collard, J. M., Giles-Vernick, T., Doria, M., Vigan-Womas, I., Rubbo, P. A., Etienne, A., Andriatahirintsoa, E. J., Kapel, N., Brown, E., Huus, K. E., Duffy, D., Finlay, B. B., Hasan, M., Hunald, F. A., Robinson, A., Manirakiza, A., … Sansonetti, P. J. (2018b). Identifying the etiology and pathophysiology underlying stunting and environmental enteropathy: Study protocol of the AFRIBIOTA project. BMC Pediatrics, 18(1), 236.
WHO Multicentre Growth Reference Study Group. (2007). Standards: Head circumference-for-age, arm circumference-for-age, triceps skinfold-for-age and subscapular skinfold-for-age: Methods and development. World Health Organization.
World Health Organization. (2007a). Assessment of iodine deficiency disorders and monitoring their elimination: A guide for programme managers. WHO Press.
World Health Organization (2007b). Indicators for assessing infant and young child feeding practices: Conclusions of a consensus meeting held. World Health Organization, Washington, 6–8 November 2007
Acknowledgements
The epidemiological part of AFRIBIOTA was funded by the Total Foundation and the Fondation Petram. PV was supported by an Early Postdoctoral Fellowship (Grant No. P2EZP3_152159), an Advanced Postdoctoral Fellowship (Grant No. P300PA_177876) as well as a Return Grant (Grant No. P3P3PA_17877) from the Swiss National Science Foundation, a Roux-Cantarini Fellowship (2016) and a L’Oréal-UNESCO for Women in Science France Fellowship (2017). PJS is a HHMI Senior Foreign Scholar. The funders had no role in study design or collection, analysis, and interpretation of data and in writing this manuscript.
AFRIBIOTA Investigators: Laurence Barbot-Trystram, Hôpital Pitié-Salpêtrière, Paris, France; Robert Barouki, Hôpital Necker-Enfants maladies, Paris, France; Alexandra Bastaraud, Institut Pasteur de Madagascar, Antananarivo, Madagascar; Jean-Marc Collard, Institut Pasteur de Madagascar, Antananarivo, Madagascar; Maria Doria, Institut Pasteur, Paris, France; Aurélie Etienne, Institut Pasteur, Paris, France/Institut Pasteur de Madagascar, Madagascar; Serge Ghislain Djorie, Institut Pasteur de Bangui, Bangui, Central African Republic; Tamara Giles-Vernick, Institut Pasteur, Paris, France; Bolmbaye Privat Gondje, Complexe Pédiatrique de Bangui, Bangui, Central African Republic; Jean-Chrysostome Gody, Complexe Pédiatrique de Bangui, Bangui, Central African Republic; Francis Allen Hunald, Service de Chirurgie pédiatrique, Centre Hospitalier Universitaire Joseph Ravoahangy Andrianavalona (CHU-JRA), Antananarivo, Madagascar; Nathalie Kapel, Hôpital Pitié-Salpêtrière, Paris, France; Jean-Pierre Lombart, Institut Pasteur de Bangui, Bangui, Central African Republic; Alexandre Manirakiza, Institut Pasteur de Bangui, Bangui, Central African Republic; Synthia Nazita Nigatoloum, Complexe Pédiatrique de Bangui, Bangui, Central African Republic; Lisette Raharimalala, Centre de Santé Materno-Infantile, Tsaralalana, Antananarivo, Madagascar; Maheninasy Rakotondrainipiana, Institut Pasteur de Madagascar, Antananarivo, Madagascar; Rindra Randremanana, Institut Pasteur de Madagascar, Antananarivo, Madagascar; Harifetra Mamy Richard Randriamizao, Centre Hospitalier Universitaire Joseph Ravoahangy Andrianavalona (CHU-JRA), Antananarivo, Madagascar; Frédérique Randrianirina, Institut Pasteur de Madagascar, Antananarivo, Madagascar; Annick Robinson, Centre Hospitalier Universitaire Mère Enfant de Tsaralalana, Antananarivo, Madagascar; Pierre-Alain Rubbo, Institut Pasteur de Bangui, Bangui, République Centrafricaine; Philippe Sansonetti, Institut Pasteur, Paris, France; Laura Schaeffer, Institut Pasteur, Paris, France; Ionela Gouandjika-Vassilache, Institut Pasteur de Bangui, Bangui, République Centrafricaine; Pascale Vonaesch, Institut Pasteur, Paris, France; Sonia Sandrine Vondo, Complexe Pédiatrique de Bangui, Bangui, Central African Republic; Inès Vigan-Womas, Institut Pasteur de Madagascar, Antananarivo, Madagascar. We wish to thank further all families and fieldworkers who contributed to this study.
Author information
Authors and Affiliations
Consortia
Contributions
PV, PJS, JCG and RVR designed the research. PV, KJEK, MR, PVA, RR, BPG, SN, SSV, AE, FR, AB and SGD conducted the data collection. AR, FAH, LR and JCG supervised data acquisition in the hospital. LS constructed the database. PV and LS performed the statistical analyses. TGV provided anthropological data for interpretation of the results. PV wrote the manuscript and all co-authors critically read and corrected the final version. PV, PJS and RVR have primary responsibility for the final content.
Corresponding authors
Ethics declarations
Ethical Approval
The AFRIBIOTA study protocol was approved by the Institutional Review Board of the Institut Pasteur (2016–06/IRB) and the national ethical review boards of Madagascar (55/MSANP/CE) and the Central African Republic (173/UB/FACSS/CSCVPER/16). Legal representatives of all child participants received oral and written information about the study and provided written consent for their children to participate in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Philippe Jean Sansonetti and Rindra Vatosoa Randremanana are considered as the co-last authors.
The AFRIBIOTA investigators are listed in the acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vonaesch, P., Djorie, S.G., Kandou, K.J.E. et al. Factors Associated with Stunted Growth in Children Under Five Years in Antananarivo, Madagascar and Bangui, Central African Republic. Matern Child Health J 25, 1626–1637 (2021). https://doi.org/10.1007/s10995-021-03201-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10995-021-03201-8