Abstract
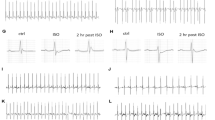
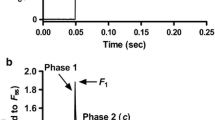
In both humans and mice, the Glu-99-Lys (E99K) mutation in the cardiac actin gene (ACTC) results in little understood apical hypertrophic cardiomyopathy (AHCM). To determine how cross-bridge kinetics change with AHCM development, we applied sinusoidal length perturbations to skinned papillary muscle fibres from 2- and 5-month old E99K transgenic (Tg) and non-transgenic (NTg) mice, and studied tension and its transients. These age groups were chosen because our preliminary studies indicated that AHCM develops with age. Fibres from 5-month old E99K mice showed significant decreases in tension, stiffness, the rate of the medium-speed exponential process and its magnitude compared to non-transgenic control. The nucleotide association constants increased with age, and they were significantly larger in E99K compared to NTg. However, there were no large differences in the rates of the cross-bridge detachment step, the rates of the force generation step, or the phosphate association constant. Our result on force/cross-bridge demonstrates that the decreased active tension of E99K fibres was caused by a decreased amount of force generated per each cross-bridge. The effects were generally less or insignificant at 2 months. A pCa-tension study showed increased Ca2+-sensitivity (pCa50) with age in both the E99K and NTg sample groups, and pCa50 was significantly larger (but only for 0.05–0.06 pCa units) in E99K than in NTg groups. A significant decrease in cooperativity (nH) was observed only in 5-month old E99K mice. We conclude that the AHCM-causing ACTC E99K mutation is associated with progressive alterations in biomechanical parameters, with changes smaller at 2 months but larger at 5 months, correlating with the development of AHCM.











Similar content being viewed by others
Abbreviations
- 2πb :
-
Apparent rate constant of the delayed tension (exponential process B)
- 2πc :
-
Apparent rate constant of fast tension recovery (exponential process C)
- ACTA1 :
-
Skeletal actin gene
- ACTC :
-
Cardiac actin gene
- AHCM:
-
Apical HCM
- B :
-
Magnitude of exponential process B
- C :
-
Magnitude of exponential process C
- CK:
-
Creatine kinase
- D :
-
MgADP or its concentration
- E99K:
-
Glu-99-Lys
- f :
-
Frequency of length oscillation
- HCM:
-
Hypertrophic cardiomyopathy
- K 0 :
-
MgADP association constant
- K 1 :
-
MgATP association constant
- k 2 :
-
Rate constant of the cross-bridge detachment step 2
- k − 2 :
-
Rate constant of the reversal of step 2
- K 2 :
-
Equilibrium constant of step 2 (= k2/k− 2)
- k 4 :
-
Rate constant of force generation step 4 (isomerization of the AM.ADP.Pi state)
- k − 4 :
-
Rate constant of the reversal of step 4
- K 4 :
-
Equilibrium constant of step 4 (= k4/k− 4)
- K 5 :
-
Phosphate association constant
- k TR :
-
The rate of tension redevelopment
- LV:
-
Left ventricle
- n H :
-
Cooperativity
- NTg:
-
Non-transgenic
- P :
-
Pi (phosphate) or its concentration
- PCr:
-
Phosphocreatine
- pCa50 :
-
Ca2+ sensitivity
- RV:
-
Right ventricle
- S :
-
MgATP or its concentration
- SCD:
-
Sudden cardiac death
- T 5 :
-
Tension per cross-bridge supported by the AM*ADP.Pi state
- Y(f):
-
Complex modulus
- ΔL :
-
Length change
References
AHA (2009) Heart disease and stroke statistics. American Heart Association, Dallas
Bai F, Caster HM, Dawson JF, Kawai M (2015) The immediate effect of HCM causing actin mutants E99K and A230V on actin-Tm-myosin interaction in thin-filament reconstituted myocardium. J Mol Cell Cardiol 79C:123–132
Behrmann E, Muller M, Penczek PA, Mannherz HG, Manstein DJ, Raunser S (2012) Structure of the rigor actin-tropomyosin-myosin complex. Cell 150:327–338
Bergen HR, Ajtai K, Burghardt TP, Nepomuceno AI, Muddiman DC (2003) Mass spectral determination of skeletal/cardiac actin isoform ratios in cardiac muscle. Rapid Commun Mass Spectrom 17:1467–1471
Bookwalter CS, Trybus KM (2006) Functional consequences of a mutation in an expressed human a-cardiac actin at a site implicated in familial hypertrophic cardiomyopathy. J Biol Chem 281:16777–16784
Bremel RD, Weber A (1972) Cooperation within actin filament in vertebrate skeletal muscle. Nat New Biol 238:97–101
D’Andrea A, Caso P, Bossone E, Scarafile R, Riegler L, Di Salvo G, Gravino R, Cocchia R, Castaldo F, Salerno G, Golia E, Limongelli G, De Corato G, Cuomo S, Pacileo G, Russo MG, Calabro R (2010) Right ventricular myocardial involvement in either physiological or pathological left ventricular hypertrophy: an ultrasound speckle-tracking two-dimensional strain analysis. Eur J Echocardiogr 11:492–500
Dahari M, Dawson JF (2015) Do cardiac actin mutations lead to altered actomyosin interactions? Biochem Cell Biol 93:330–334
Dantzig J, Goldman Y, Millar NC, Lacktis J, Homsher E (1992) Reversal of the cross-bridge force-generating transition by the photogeneration of phosphate in rabbit psoas muscle fibers. J Physiol 451:247–278
Durrwang U, Fujita-Becker S, Erent M, Kull FJ, Tsiavaliaris G, Geeves MA, Manstein DJ (2006) Dictyostelium myosin-IE is a fast molecular motor involved in phagocytosis. J Cell Sci 119:550–558
Force T, Bonow RO, Houser SR, Solaro RJ, Hershberger RE, Adhikari B, Anderson ME, Boineau R, Byrne BJ, Cappola TP, Kalluri R, LeWinter MM, Maron MS, Molkentin JD, Ommen SR, Regnier M, Tang WH, Tian R, Konstam MA, Maron BJ, Seidman CE (2010) Research priorities in hypertrophic cardiomyopathy: report of a Working Group of the National Heart, Lung, and Blood Institute. Circulation 122:1130–1133
Fortune NS, Geeves MA, Ranatunga KW (1991) Tension responses to rapid pressure release in glycerinated rabbit muscle fibers. Proc Natl Acad Sci (USA) 88:7323–7327
Furch M, Geeves MA, Manstein DJ (1998) Modulation of actin affinity and actomyosin adenosine triphosphatase by charge changes in the myosin motor domain. Biochemistry 37:6317–6326
Geeves MA, Holmes KC (1999) Structural mechanism of muscle contraction. Ann Rev Biochem 68:687–728
Holmes KC, Schroder RR, Sweeney HL, Houdusse A (2004) The structure of the rigor complex and its implications for the power stroke. Philos Trans R Soc Lond B Biol Sci 359:1819–1828
Huxley HE, Stewart A, Sosa H, Irving T (1994) X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J 67:2411–2421
Kaski JP, Syrris P, Esteban MT, Jenkins S, Pantazis A, Deanfield JE, McKenna WJ, Elliott PM (2009) Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet 2:436–441
Kawai M (2003) What do we learn by studying the temperature effect on isometric tension and tension transients in mammalian striated muscle fibres? J Muscle Res Cell Motil 24:127–138
Kawai M, Brandt PW (1980) Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Mot 1:279–303
Kawai M, Halvorson H (1989) Role of MgATP and MgADP in the crossbridge kinetics in chemically skinned rabbit psoas fibers. Study of a fast exponential process C. Biophys J 55:595–603
Kawai M, Halvorson HR (1991) Two step mechanism of phosphate release and the mechanism of force generation in chemically skinned fibers of rabbit psoas. Biophys J 59:329–342
Kawai M, Zhao Y (1993) Cross-bridge scheme and force per cross-bridge state in skinned rabbit psoas muscle fibers. Biophys J 65:638–651
Kawai M, Saeki Y, Zhao Y (1993) Cross-bridge scheme and the kinetic constants of elementary steps deduced from chemically skinned papillary and trabecular muscles of the ferret. Circ Res 73:35–50
Lorenz M, Holmes KC (2010) The actin-myosin interface. Proc Natl Acad Sci USA 107:12529–12534
Lu X, Bryant MK, Bryan KE, Rubenstein PA, Kawai M (2005) Role of the N-terminal negative charges of actin in force generation and cross-bridge kinetics in reconstituted bovine cardiac muscle fibres. J Physiol 564:65–82
Marston SB (2011) How do mutations in contractile proteins cause the primary familial cardiomyopathies? J Cardiovasc Transl Res 4:245–255
Miller CJ, Wong WW, Bobkova E, Rubenstein PA, Reisler E (1996) Mutational analysis of the role of the N terminus of actin in actomyosin interactions. Comparison with other mutant actins and implications for the cross-bridge cycle. Biochemistry 35:16557–16565
Mogensen J, Klausen IC, Pedersen AK, Egeblad H, Bross P, Kruse TA, Gregersen N, Hansen PS, Baandrup U, Borglum AD (1999) Alpha-cardiac actin is a novel disease gene in familial hypertrophic cardiomyopathy. J Clin Investig 103:R39–R43
Mogensen J, Perrot A, Andersen PS, Havndrup O, Klausen IC, Christiansen M, Bross P, Egeblad H, Bundgaard H, Osterziel KJ, Haltern G, Lapp H, Reinecke P, Gregersen N, Borglum AD (2004) Clinical and genetic characteristics of alpha cardiac actin gene mutations in hypertrophic cardiomyopathy. J Med Genet 41:e10
Mozaffarian D, Caldwell JH (2001) Right ventricular involvement in hypertrophic cardiomyopathy: a case report and literature review. Clin Cardiol 24:2–8
Mundia MM, Demers RW, Chow ML, Perieteanu AA, Dawson JF (2012) Subdomain location of mutations in cardiac actin correlate with type of functional change. PLoS One 7:e36821
Murphy KP, Zhao Y, Kawai M (1996) Molecular forces involved in force generation during skeletal muscle contraction. J Exp Biol 199:2565–2571
Nowak KJ, Ravenscroft G, Jackaman C, Filipovska A, Davies SM, Lim EM, Squire SE, Potter AC, Baker E, Clément S, Sewry CA, Fabian V, Crawford K, Lessard JL, Griffiths LM, Papadimitriou JM, Shen Y, Morahan G, Bakker AJ, Davies KE, Laing NG (2009) Rescue of skeletal muscle α-actin–null mice by cardiac (fetal) α-actin. J Cell Biol 185:903–915
Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT (1998) Actin mutations in dilated cardiomyopathy, a heritable form of heart failure. Science 280:750–752
Olson TM, Doan TP, Kishimoto NY, Whitby FG, Ackerman MJ, Fananapazir L (2000) Inherited and de novo mutations in the cardiac actin gene cause familial hypertrophic cardiomyopathy. J Mol Cell Cardiol 32:1687–1694
Opie LH, Mansford KR, Owen P (1971) Effects of increased heart work on glycolysis and adenine nucleotides in the perfused heart of normal and diabetic rats. Biochem J 124:475–490
Perhonen MA, Franco F, Lane LD, Buckey JC, Blomqvist CG, Zerwekh JE, Peshock RM, Weatherall PT, Levine BD (2001) Cardiac atrophy after bed rest and spaceflight. J Appl Physiol 91:645–653
Rall JA, Woledge RC (1990) Influence of temperature on mechanics and energetics of muscle contraction. Am J Physiol 259:R197-203
Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA (1993) Structure of the actin-myosin complex and its implications for muscle contraction. Science 261:58–65
Roth K, Hubesch B, Meyerhoff DJ, Naruse S, Gober JR, Lawry TJ, Boska MD, Matson GB, Weiner MW (1989) Noninvasive quantitation of phosphorus metabolites in human tissue by NMR spectroscopy. J Magn Reson 81:299–311
Rowlands CT, Owen T, Lawal S, Cao S, Pandey SP, Yang H-Y, Song W, Wilkinson R, Alvarez-Laviada A, Gehmlich K, Marston SB, MacLeod KT (2017) Age- and strain-related aberrant Ca2+ release is associated with sudden cardiac death in the ACTC E99K mouse model of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 313:H1213–H1226
Schober KE, Savino SI, Yildiz V (2016) Right ventricular involvement in feline hypertrophic cardiomyopathy. J Vet Cardiol 18:297–309
Seidman CE, Seidman JG (2011) Identifying sarcomere gene mutations in hypertrophic cardiomyopathy: a personal history. Circ Res 108:743–750
Semsarian C, Ingles J, Maron MS, Maron BJ (2015) New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 65:1249–1254
Song W, Dyer E, Stuckey DJ, Copeland O, Leung MC, Bayliss C, Messer A, Wilkinson R, Tremoleda JL, Schneider MD, Harding SE, Redwood CS, Clarke K, Nowak K, Monserrat L, Wells D, Marston SB (2011) Molecular mechanism of the E99K mutation in cardiac actin (ACTC Gene) that causes apical hypertrophy in man and mouse. J Biol Chem 286:27582–27593
Song W, Vikhorev PG, Kashyap MN, Rowlands C, Ferenczi MA, Woledge RC, MacLeod K, Marston S, Curtin NA (2013) Mechanical and energetic properties of papillary muscle from ACTC E99K transgenic mouse models of hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 304:H1513–H1524
Sutoh K (1983) Mapping of actin-binding sites on the heavy chain of myosin subfragment 1. Biochemistry 22:1579–1585
Taylor EW (1979) Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem 6:103–164
Thirlwell H, Corrie JE, Reid GP, Trentham DR, Ferenczi MA (1994) Kinetics of relaxation from rigor of permeabilized fast-twitch skeletal fibers from the rabbit using a novel caged ATP and apyrase. Biophys J 67:2436–2447
Thirlwell H, Sleep JA, Ferenczi MA (1995) Inhibition of unloaded shortening velocity in permeabilized muscle fibres by caged ATP compounds. J Muscle Res Cell Motil 16:131–137
Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ (2003) Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation 108:445–451
Vandekerckhove J, Bugaisky G, Buckingham M (1986) Simultaneous expression of skeletal muscle and heart actin proteins in various striated muscle tissues and cells. A quantitative determination of the two actin isoforms. J Biol Chem 261:1838–1843
Wakabayashi K, Sugimoto Y, Tanaka H, Ueno Y, Takezawa Y, Amemiya Y (1994) X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J 67:2422–2435
Wang L, Kawai M (2013) A re-interpretation of the rate of tension redevelopment (kTR) in active muscle. J Muscle Res Cell Motil 34:407–415
Wang G, Ding W, Kawai M (1999) Does thin filament compliance diminish the cross-bridge kinetics? A study in rabbit psoas fibers. Biophys J 76:978–984
Wang L, Muthu P, Szczesna-Cordary D, Kawai M (2013) Diversity and similarity of motor function and cross-bridge kinetics in papillary muscles of transgenic mice carrying myosin regulatory light chain mutations D166Vand R58Q. J Mol Cell Cardiol 62:153–163
Wang L, Sadayappan S, Kawai M (2014) Cardiac myosin binding protein C phosphorylation affects cross-bridge cycle’s elementary steps in a site-specific manner. PLoS One 9:1–21
Wang L, Bahadir A, Kawai M (2015) High ionic strength depresses muscle contractility by decreasing both force per cross-bridge and the number of strongly attached cross-bridges. J Muscle Res Cell Motil 36:227–241
Wannenburg T, Heijne GH, Geerdink JH, Van-Den-Dool HW, Janssen PM, DeTombe PP (2000) Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. Am J Physiol 279:H779-H790
Zhao Y, Kawai M (1994) Kinetic and thermodynamic studies of the cross-bridge cycle in rabbit psoas muscle fibers. Biophys J 67:1655–1668
Zhao Y, Swamy PMG, Humphries KA, Kawai M (1996) The effect of partial extraction of troponin C on the elementary steps of the cross-bridge cycle in rabbit psoas fibers. Biophys J 71:2759–2773
Acknowledgements
The authors would like to thank Professor Steven Marston (National Heart and Lung Institute, Imperial College London, London, UK) who developed the Tg mouse model ACTC E99K and made our collaboration possible. The authors also would like to thank Dr. Amy Li in University of Sydney for developing a technique for freezing and thawing muscle samples without causing much damage, and teaching the technique to us. This work was supported by grants from the Natural Science Foundation of Jiangsu Province of China BK20150353 (LW), the National Institutes of Health HL070041 (MK), and The American Heart Association 13GRNT16810043 (MK). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the funding organizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest.
Rights and permissions
About this article
Cite this article
Wang, L., Bai, F., Zhang, Q. et al. Development of apical hypertrophic cardiomyopathy with age in a transgenic mouse model carrying the cardiac actin E99K mutation. J Muscle Res Cell Motil 38, 421–435 (2017). https://doi.org/10.1007/s10974-018-9492-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10974-018-9492-1