Abstract
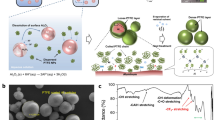
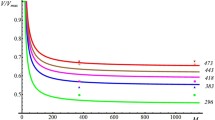
Micron-diameter aluminum particles (μAl) are highly reactive when combined with a solid oxidizer. However, μAl powder is less reactive in a gaseous air environment, where oxygen is the only available oxidizer, unless very well dispersed. While enthalpy of oxidation for Al is high, many variables influence the viability of harnessing stored chemical energy within a single Al particle whose reaction is diffusion controlled. One way to enhance Al particle reaction is to coat the particle surface with a condensed phase oxidizing agent that is in immediate contact with the particle surface to promote diffusion reactions. Fluorocarbons such as perfluorocarboxylic acids have been used to enhance Al combustion for nanoscale Al (nAl) particles because fluorinated species are also reactive with the Al2O3 passivation shell surrounding the Al core particle. This study extends previous work on nAl toward μAl particles coated with perfluorotetradecanoic acid (PFTD) (F3C(CF2)11CO2H) and then characterizes the μAl-PFTD thermal reactivity. Samples were prepared with varying PFTD concentrations ranging from 0 to 20 mass percent, and experiments were performed using thermogravimetric analysis and high-speed infrared imaging on powder samples. Results show the PFTD-coated μAl particles provide higher apparent reaction temperatures (by about 500 °C) and longer burn times at elevated temperatures (by about 20%). Higher concentrations of PFTD tend to produce more gas-generating reactions with particles ejecting from the loose powder pile, but 9% PFTD coating on μAl particles provides a good balance of stable reactivity with high-temperature reactions (~ 1800 °C) and high-temperature burn times (~ 18 s). The higher temperatures for PFTD-coated μAl particles are attributed to the increased (nearly double) energy produced in the formation of AlF3 (56.10 kJ g−1) compared to Al2O3 (30.98 kJ g−1); both of these product species are identified in XRD analysis. In addition, the formation of AlF3 may reduce the melting and phase transition temperature of Al2O3 by several hundred degrees and contribute to catalytically activating the Al reaction. Overall, coating μAl particles with a fluorinated polymer facilitates reaction in an air environment, even for small concentrations of PFTD coating.




Similar content being viewed by others
References
Pines H, WERNER OH. Alumina: catalyst and support. I. alumina, its intrinsic acidity and catalytic activity. J Am Chem Soc. 1960;82(10):2471–83.
Ma Z-L, Jia R-L, Liu C-J. Production of hydrogen peroxide from carbon monoxide, water and oxygen over alumina-supported Ni catalysts. J Mol Catal A: Chem. 2004;210(1–2):157–63.
Ku Y, Chiou H. The adsorption of fluoride ion from aqueous solution. Water Air Soil Pollut. 2002;133:349–60.
Onodera T, Nakakawaji T, Adachi K, Kurihara K, Kubo M. Tribochemical degradation of polytetrafluoroethylene catalyzed by copper and aluminum surfaces. J Phys Chem C. 2016;120(20):10857–65.
Osborne DT, Pantoya ML. Effect of Al particle size on the thermal degradation of Al/Teflon mixtures. Combust Sci Technol. 2007;179(8):1467–80.
McCollum J, Pantoya ML, Iacono ST. Catalyzing aluminum particle reactivity with a fluorine oligomer surface coating for energy generating applications. J Fluor Chem. 2015;180:265–71.
Hess A. Characterization of catalytically active sites on aluminum oxides, hydroxyfluorides, and fluorides in correlation with their catalytic behavior. J Catal. 1994;149:449–57.
van den Brand J, Blajiev O, Beentjes PCJ, Terryn H, de Wit JHW. Interaction of anhydride and carboxylic acid compounds with aluminum oxide surfaces studied using Infrared reflection absorption spectroscopy. Langmuir. 2004;20(15):6308–17.
Crouse CA, Pierce CJ, Spowart JE. Influencing solvent miscibility and aqueous stability of aluminum nanoparticles through surface functionalization with acrylic monomers. Appl Mater Interfaces. 2010;2(9):2560–9.
Crouse CA, Pierce CJ, Spowart JE. Synthesis and reactivity of aluminized fluorinated acrylic (AlFA) nanocomposites. Combust Flame. 2012;159:3199–207.
Karaman ME, Antelmi DA, Pashley RM. The production of stable hydrophobic surfaces by the adsorption of hydrocarbon and fluorocarbon carboxylic acids onto alumina substrates. Colloids Surf A. 2001;182:285–98.
Taylor CE, Schwartz DK. Octadecanoic acid self-assembled monolayer growth at sapphire surfaces. Langmuir. 2003;19:2665–72.
Kappagantula KS, Farley C, Pantoya ML, Horn J. Tuning energetic material reactivity using surface functionalization of aluminum fuels. J Phys Chem C. 2012;116(46):24469–75.
Dweck J, Aderne RS, Shanefield DJ. Aluminum nitride oxidation by simultaneous TG and DTA. J Therm Anal Calor. 2001;64:1163–9.
Jouet RJ, Carney JR, Granholm RH, Sandusky HW, Warren AD. Preparation and reactivity analysis of novel perfluoroalkyl coated aluminium nanocomposites. Mater Sci Technol. 2006;22(4):422–9.
Jouet RJ, Warren AD, Rosenberg DM, Bellitto VJ, Park K, Zachariah MR. Surface passivation of bare aluminum nanoparticles using perfluoroalkyl carboxylic acids. Chem Mater. 2005;17:2987–96.
Dikici B, Dean SW, Pantoya ML, Levitas VI, Jouet RJ. Influence of aluminum passivation on the reaction mechanism: flame propagation studies. Energy Fuels. 2009;23:4231–5.
McNesby KL, Homan BE, Benjamin RA, Boyle VM, Biss MM, Densmore JM. Near field optical characterization of explosions. Shock compression of condensed matter. AIP Conf Proc. 2017;1793:060008-1.
Densmore JM, Homan BE, Biss MM, McNesby KL. High-speed two-camera imaging pyrometer for mapping fireball temperatures. Appl Opt. 2011;50(33):6267–71.
Boboridis K, Obst AW. A high-speed four-channel Infrared pyrometer. AIP Conf Proc. 2003;684:759.
Bassett WP, Dlott DD. Multichannel emission spectrometer for high dynamic range optical pyrometry of shock-driven materials. Rev Sci Instrum. 2016;87(10):103107.
Vollmer M, Möllmann K-P. Infrared thermal imaging: fundamentals, research and applications. Weinheim: Wiley-VCH; 2018.
Krahl T, Kemnitz E. Aluminium fluoride—the strongest solid Lewis acid: structure and reactivity. Catal Sci Technol. 2017;7(4):773–96.
Riello D, Zetterstrom C, Parr C, Braulio MAL, Moreira M, Gallo JB. AlF3 reaction mechanism and its influence on α-Al2O3 mineralization. Ceram Int. 2016;42:9804–14.
Yamai I, Saito H. Vapor phase growth of alumina whiskers by hydrolysis of aluminum fluoride. J Cryst Growth. 1978;45:511–6.
Fu G, Jing W, Jian K. Influence of AlF3 and hydrothermal conditions on morphologies of α-Al2O3. Trans Nonferr Metals Soc China. 2008;18(3):743–8.
Lide DR, editor. CRC handbook of chemistry and physics. 77th ed. Boca Raton: CRC Press; 1996.
Yuan J, Liu J, Zhou Y, Zhang Y, Cen K. Thermal decomposition and combustion characteristics of Al/AP/HTPB propellant. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09297-4.
Acknowledgements
The authors are grateful for support from DTRA under award HDTRA1-15-1-0029 and partial support by ARO under award W911NF-17-10387. We would also like to thank Dr. Daniel Unruh at Texas Tech University Chemistry Department for assistance with XRD analysis and Drs. R. Carl Brothers and R. Wilson at Indian Head Naval Surface Warfare Center for preparation of the materials investigated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Campbell, L.L., Hill, K.J., Smith, D.K. et al. Thermal analysis of microscale aluminum particles coated with perfluorotetradecanoic (PFTD) acid. J Therm Anal Calorim 145, 289–296 (2021). https://doi.org/10.1007/s10973-020-09742-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-09742-4