Abstract
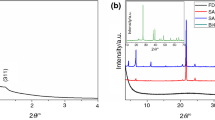
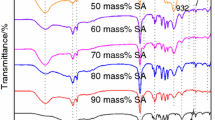
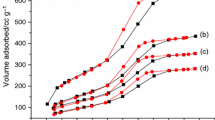
The synthesis of phase change materials based on NaCl–CaCl2 molten salt mixture and mesoporous silica was investigated. The influence of mesoporous silica porosity and salt concentration on the thermal energy storage properties of the resulting materials is discussed. The nanocomposite samples were characterized by X-ray diffraction, differential scanning calorimetry, infrared spectroscopy, thermogravimetry, scanning electron microscopy and X-ray photoelectron spectroscopy. The mesoporous silica was found to act as a reactive matrix for the molten salts. Composite samples with up 95% wt. salt can be obtained and used as shape-stabilized phase change materials. The materials have heat of fusion values of up to 60.8 J g−1 and specific heat capacity between 1.0 and 1.1 J g−1 K−1. The samples exhibit thermal stability up to 700 °C and can be used for high-temperature thermal energy storage through both latent and sensible heat storage mechanisms.








Similar content being viewed by others
References
Zhang G, Li J, Chen Y, Xiang H, Ma B, Xu Z, et al. Encapsulation of copper-based phase change materials for high temperature thermal energy storage. Sol Energy Mater Sol Cells. 2014;128:131–7. https://doi.org/10.1016/j.solmat.2014.05.012.
Kenisarin MM. High-temperature phase change materials for thermal energy storage. Renew Sustain Energy Rev. 2010;14(3):955–70. https://doi.org/10.1016/j.rser.2009.11.011.
Gil A, Medrano M, Martorell I, Lázaro A, Dolado P, Zalba B, et al. State of the art on high temperature thermal energy storage for power generation. Part 1—concepts, materials and modellization. Renew Sustain Energy Rev. 2010;14(1):31–55. https://doi.org/10.1016/j.rser.2009.07.035.
Zhang Q, Liu J. Nano liquid metal as an emerging functional material in energy management, conversion and storage. Nano Energy. 2013;2(5):863–72. https://doi.org/10.1016/j.nanoen.2013.03.002.
Schrader AJ, Muroyama AP, Loutzenhiser PG. Solar electricity via an air brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Co3O4/CoO redox reactions: thermodynamic analysis. Sol Energy. 2015;118:485–95. https://doi.org/10.1016/j.solener.2015.05.045.
Liu M, Saman W, Bruno F. Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renew Sustain Energy Rev. 2012;16(4):2118–32. https://doi.org/10.1016/j.rser.2012.01.020.
Wu S, Ma X, Peng D, Bi Y. The phase change property of lauric acid confined in carbon nanotubes as nano-encapsulated phase change materials. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7906-3.
Mitran R-A, Berger D, Matei C. Improving thermal properties of shape-stabilized phase change materials containing lauric acid and mesocellular foam silica by assessing thermodynamic properties of the non-melting layer. Thermochim Acta. 2018;660:70–6. https://doi.org/10.1016/j.tca.2017.12.019.
Liu S, Ma G, Xie S, Jia Y, Sun J, Jing Y. Diverting the phase transition behaviour of adipic acid via mesoporous silica confinement. RSC Adv. 2016;6(113):111787–96. https://doi.org/10.1039/c6ra23498d.
Deng S, Wang D, Wang X, Wei Y, Waterhouse GI, Lan XZ. Effect of nanopore confinement on the thermal and structural properties of heneicosan. Thermochim Acta. 2018;664:57–63.
Tran N, Zhao W, Carlson F, Davidson JH, Stein A. Metal nanoparticle–carbon matrix composites with tunable melting temperature as phase-change materials for thermal energy storage. ACS Appl Nano Mater. 2018;1(4):1894–903.
Fernández AI, Barreneche C, Belusko M, Segarra M, Bruno F, Cabeza LF. Considerations for the use of metal alloys as phase change materials for high temperature applications. Sol Energy Mater Sol Cells. 2017;171:275–81. https://doi.org/10.1016/j.solmat.2017.06.054.
Aftab W, Huang X, Wu W, Liang Z, Mahmood A, Zou R. Nanoconfined phase change materials for thermal energy applications. Energy Environ Sci. 2018. https://doi.org/10.1039/c7ee03587j.
Mitran R-A, Berger D, Matei C. Phase change materials based on mesoporous silica. Curr Org Chem. 2018;22(27):2644–63. https://doi.org/10.2174/1385272822666180827125651.
Majda D, Korzeniowska A, Makowski W, Michalik-Zym A, Napruszewska BD, Zimowska M, et al. Thermoporosimetry of n-alkanes for characterization of mesoporous SBA-15 silicas—refinement of methodology. Microporous Mesoporous Mater. 2016;222:33–43. https://doi.org/10.1016/j.micromeso.2015.10.002.
Chen D, Chen Y, Guo X, Tao W, Wang J, Gao S, et al. Mesoporous silica nanoparticles with wrinkled structure as the matrix of myristic acid for the preparation of a promising new shape-stabilized phase change material via simple method. RSC Adv. 2018;8(60):34224–31. https://doi.org/10.1039/c8ra06536e.
Mitran R-A, Nastase S, Matei C, Berger D. Tailoring the dissolution rate enhancement of aminoglutethimide by functionalization of MCM-41 silica: a hydrogen bonding propensity approach. RSC Adv. 2015;5(4):2592–601. https://doi.org/10.1039/c4ra11224e.
Linares N, Silvestre-Albero AM, Serrano E, Silvestre-Albero J, Garcia-Martinez J. Mesoporous materials for clean energy technologies. Chem Soc Rev. 2014;43(22):7681–717. https://doi.org/10.1039/c3cs60435g.
Qian T, Li J, Min X, Deng Y, Guan W, Ning L. Radial-like mesoporous silica sphere: a promising new candidate of supporting material for storage of low-, middle-, and high-temperature heat. Energy. 2016;112:1074–83. https://doi.org/10.1016/j.energy.2016.07.023.
Mitran RA, Berger D, Munteanu C, Matei C. Evaluation of different mesoporous silica supports for energy storage in shape-stabilized phase change materials with dual thermal responses. J Phys Chem C. 2015;119(27):15177–84. https://doi.org/10.1021/acs.jpcc.5b02608.
Fan J, Yu C, Gao F, Lei J, Tian B, Wang L, et al. Cubic mesoporous silica with large controllable entrance sizes and advanced adsorption properties. Angew Chem. 2003;115(27):3254–8. https://doi.org/10.1002/ange.200351027.
Grudzien RM, Grabicka BE, Jaroniec M. Adsorption studies of thermal stability of SBA-16 mesoporous silicas. Appl Surf Sci. 2007;253(13):5660–5. https://doi.org/10.1016/j.apsusc.2006.12.033.
Palubinskaitė D, Kantautas A. Influence of tribomechanical milling and activation of primary mixtures on the synthesis of calcium silicate hydrates. Mater Sci Poland. 2006;24(2/1):396–403.
Mocioiu OC, Mocioiu A-M, Marin A, Zaharescu M. Study of historical lead silicate glasses and their preservation by silica coating. Ceram Int. 2017;43(1):77–83. https://doi.org/10.1016/j.ceramint.2016.09.055.
Zaharescu M, Mocioiu OC, Andronescu C. Influence of CaO addition on the structure and properties of the glasses in the SiO2–PbO–Na2O system. J Optoelectron Adv Mater. 2008;10(6):1315–9.
Yu P, Kirkpatrick RJ, Poe B, McMillan PF, Cong X. Structure of calcium silicate hydrate (C–S–H): near-, mid-, and far-infrared spectroscopy. J Am Ceram Soc. 1999;82(3):742–8. https://doi.org/10.1111/j.1151-2916.1999.tb01826.x.
Hugo KC. Confinement effects on freezing and melting. J Phys: Condens Matter. 2001;13(11):R95.
Redkin AA, Zaikov YP, Korzun IV, Reznitskikh OG, Yaroslavtseva TV, Kumkov SI. Heat capacity of molten halides. J Phys Chem B. 2015;119(2):509–12. https://doi.org/10.1021/jp509932e.
Acknowledgements
The financial support of the Romanian project PN-III-P1-1.1-TE-2016-0520, No. 95/2018 is greatly appreciated. The authors are grateful to Dr. Cornel Munteanu for performing the TEM measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mitran, RA., Petrescu, S., Şomǎcescu, S. et al. Nanocomposite phase change materials based on NaCl–CaCl2 and mesoporous silica. J Therm Anal Calorim 138, 2555–2563 (2019). https://doi.org/10.1007/s10973-019-08489-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08489-x