Abstract
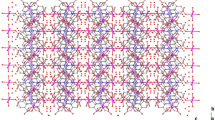
Single crystals of the polymeric title complex tetraaquadinicotinatostrontium (SPYC) were successfully grown by a simple and efficient method—the gel encapsulation technique. Sodium metasilicate was used as the gel medium for this purpose. The crystal structure of the complex indicates that it belongs to triclinic system with space group \(P\overline{1}\). The packing diagram of the complex shows uniformly arranged ‘χ’-shaped hydrophobic channels. The thermal decomposition of the crystals have been studied by thermogravimetric analysis at four different heating rates and kinetic parameters including apparent activation energy (Ea), and pre-exponential factor (log A) were calculated by Kissinger, Ozawa and Flynn–Wall–Ozawa methods. Analytical techniques including FT-IR, CHN, powder and single-crystal X-ray diffraction were also carried out to characterize the grown crystals. The solid-state luminescent properties of free ligand and complex were also investigated at room temperature.



















Similar content being viewed by others
References
Zhang M, Bosch M, Gentle T III, Zhou H. Rational design of metal-organic frameworks with anticipated porosities and functionalities. Cryst Eng Commun. 2014;16:4069–83.
Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. The chemistry and applications of metal-organic frameworks. Science. 2013;341:1230444.
Rao CNR, Natarajan S, Vaidhyanathan R. Metal Carboxylates with Open architectures. Angew Chem Int Ed. 2004;43:1466–96.
Kuppler RJ, Timmons DJ, Fang QR, Li JR, Makal TA, Young MD, Yuan D, Zhao D, Zhuang W, Zhou HC. Potential applications of metal-organic frameworks. Coord Chem Rev. 2009;253:3042–66.
Kim J, Chen B, Reineke TM, Li H, Eddaoudi M, Moler DB, O′Keeffe M, Yaghi OM. Assembly of metal-organic frameworks from large organic and inorganic secondary building units: new examples and simplifying principles for complex structures. J Am Chem Soc. 2001;123:8239–47.
Łyszczek R. Synthesis, structure, thermal and luminescent behavior of lanthanide-Pyridine-3,5-dicarboxylate frameworks series. Thermochim Acta. 2010;509:120–7.
Łyszczek R, Mazur L. Polynuclear complexes constructed by lanthanides and pyridine-3,5-dicarboxylate ligand: structures, thermal and luminescent properties. Polyhedron. 2012;41:7–19.
Zhao YH, Su ZM, Fu YM, Shao KZ, Li P, Wang Y, Hao XR, Zhu DX, Liu SD. Syntheses and characterizations of four metal coordination polymers constructed by the pyridine-3,5-dicarboxylate ligand. Polyhedron. 2008;27:583–92.
Mathew V, Jacob S, Mahadevan CK, Abraham KE. A study of thermal, dielectric and magnetic properties of strontium malonate crystals. Phys B. 2012;407:222–6.
Chen Y, She S, Gao Q, Gao D, Wang D, Li Y, Liu W, Li W. Synthesis, structures and properties of the first series of SrII-MII (M = Cu Co, Ni and Zn) coordination polymers based on pyridine-2,5-dicarboxylic acid. Cryst Eng Commun. 2014;16:1091–102.
Liu GX, Ren XM, Xu H, Nishihara S, Huang RY. A 3D Gd–Ag coordination polymer constructed from pyridine-3,5-dicarboxylic acid: synthesis, crystal structure and magnetic properties. Inorg Chem Commun. 2009;12:895–7.
Li D, Zhong GQ. Synthesis, crystal structure, and thermal decomposition of the cobalt(II) complex with 2-Picolinic Acid. Sci World J. 2014;641608:7.
Shi FN, Cunha-Silva L, Trindade T, Paz FAA, Rocha J. Three-dimensional lanthanide-organic frameworks based on di-, tetra-, and hexameric clusters. Cryst Growth Des. 2009;9:2098–109.
Remya Nair M, Sudarsanakumar MR, Suma S, Prathapachandra Kurup MR. Crystal structure and characterization of a novel luminescent 2D metal-organic framework, poly[aquaitaconatocalcium(II)] possessing an open framework structure with hydrophobic channels. J Mol Struct. 2016;1105:316–21.
Vyazovkin S, Burnham AK, Criado JM, Pérez-Maqueda LA, Popescu C, Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta. 2011;520:1–19.
Sangeetha V, Gayathri K, Krishnan P, Sivakumar N, Kanagathara N, Anbalagan G. Growth, structural, crystallization, thermal decomposition and dielectric behavior of melaminium bis(hydrogen oxalate) single crystal. J Therm Anal Calorim. 2014;117:307–18.
Aghabozorg H, Nemati A, Derikvand Z, Ghadermazi M, Daneshvar S. Poly[tetraaqua-l3-pyridine-3,5- dicarboxylato-strontium(II)]. Acta Cryst. 2008;E64:m376.
Dhanya VS, Sudarsanakumar MR, Suma S, Prathapachandra Kurup MR, Sithambaresan M, Sunalya Roy M. Spectral, thermal, structural, dielectric and microhardness studies of gel grown diaquasuccinatocadmium(II) hemihydrate. Spectrochim Acta Part A. 2012;93:295–9.
BRUKER, APEX2, SAINT, XPREP, Bruker AXS Inc. Madison, Wisconsin, USA 2004.
Altormare A, Cascarano G, Giacovazzo G, Guagliardi G. Completion and refinement of crystal-structures with SIR92. J Appl Cryst. 1993;26:343–50.
Sheldrick GM. SHELXL 97 Program for Crystal Structure Refinement. Gottingen: University of Gottingen; 1997.
Brandenburg K, DIAMOND Version 3.1f. Crystal Impact GbR, Bonn, 2008.
Akhbari K, Morsali A. Spectroscopic, thermal, fluorescence and structural studies of new Tl-I pyridine dicarboxylate complexes [Tl-2(py-2,5-dc)] and [T1(2)(py-3,5-dc)]. J Mol Struct. 2008;878:65–70.
Prasanna S, Bijini BR, Rajendra Babu K, Eapen SM, Deepa M, Nair CMK. Growth and characterisation of a novel polymer of manganese(II) nicotinate single crystal. J Cryst Growth. 2011;333:36–9.
Zang Q, Zhong G-Q, Wang M-L. A copper(II) complex with pyridine-2,6-dicarboxylic acid: synthesis, characterization, thermal decomposition, bioactivity and interactions with herring sperm DNA. Polyhedron. 2015;100:223–30.
Herbert Roesky W, Andruh M. The interplay of coordinative, hydrogen bonding and π–π stacking interactions in sustaining supramolecular solid-state architectures. A study case of bis(4-pyridyl)- and bis(4-pyridyl-N-oxide) tectons. Coord Chem Rev. 2003;236:91–119.
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Cryst B. 2002;58(3-1):389–97.
Sahu J, Aijaz A, Xu Q, Bharadwaj PK. A three-dimensional pillared-layer metal-organic framework: synthesis, structure and gas adsorption studies. Inorg Chim Acta. 2015;430:193–8.
Deng M, Yang P, Liu X, Xia B, Chen Z, Ling Y, Weng L, Zhou Y, Sun J. End–end connection pattern of trinuclear-triangular copper cluster for construction of two metal-organic frameworks: syntheses, structures, magnetic and gas adsorption properties. Cryst Growth Des. 2015;15:1526–34.
Świderski G, Kalinowska M, Rusinek I, Samsonowicz M, Rzączyńska Z, Lewandowski W. Spectroscopic (IR, Raman) and thermogravimetric studies of 3d-metal cinchomeronates and dinicotinates. J Therm Anal Calorim. 2016;126:1521–32.
Boonchom B. Kinetic and thermodynamic studies of MgHPO4 3H2O by non-isothermal decomposition data. J Therm Anal Calorim. 2009;98:863–71.
Chaiyo N, Muanghlua R, Niemcharoen S, Boonchom B, Seeharaj P, Vittayakorn N. Non-isothermal kinetics of the thermal decomposition of sodium oxalate Na2C2O4. J Therm Anal Calorim. 2011;107:1023–9.
Kissinger H. Variation of peak temperature with heating rate in different thermal analysis. J Res Natl Bur Stand. 1956;57(4):217–21.
Blaine L, Kissinger H. Homer Kissinger and the Kissinger equation. Thermochim Acta. 2012;540:1–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Matečić Mušanić S, Fiamengo Houra I, Sućeska M. Applicability of non-isothermal DSC and Ozawa method for studying kinetics of double base propellant decomposition. Central Eur J Energ Mater. 2010;7(3):233–51.
Hamed MNH, Kamal R. The effect of particle size on the kinetics of thermal decomposition of Co(C2O4) 2H2O nanopowders under non-isothermal conditions. J Therm Anal Calorim. 2016;123:675–86.
Lin W-C, Yu W-L, Liu S-H, Huang S-Y, Hou H-Y, Shu C-M. Thermal hazard analysis and combustion characteristics of four imidazolium nitrate ionic liquids. J Therm Anal Calorim. 2018;133:683–93.
Trache D, Maggi F, Palmucci I, DeLuca LT. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J Therm Anal Calorim. 2018;132:1601–15.
Uzun N, Çolak AT, Emen FM, Çılgı GK. The thermal and detailed kinetic analysis of dipicolinate complexes. J Therm Anal Calorim. 2016;124:1735–44.
Balboul BAA, El-Roudi AM, Samir E, Othman AG. Non-isothermal studies of the decomposition course of lanthanum oxalate decahydrate. Thermochim Acta. 2002;387:109–14.
Brown ME, Dollimore D. Galeway AK. In: Banford CH, Tipper CFH, editors. Reactions in solid state. Amsterdam: Elsevier; 1980.
Koga N. Ozawa’s kinetic method for analyzing thermoanalytical curves. J Therm Anal Calorim. 2013;113:1527–41.
Sivakumar N, Kanagathara N, Gayathri K, Krishnan P, Anbalagan G. Synthesis, thermal decomposition and dielectric behavior of bis(thiourea)silver(I)nitrate. J Therm Anal Calorim. 2014;115:1295–301.
Muraleedharan K, Kripa S. DSC kinetics of the thermal decomposition of copper(II) oxalate by isoconversional and maximum rate (peak) methods. J Therm Anal Calorim. 2014;115:1969–78.
John J, Sudha Devi R, Balachandran S, Dinesh Babu KV. Synthesis, spectral characterization and thermal analysis of rubrocurcumin and its analogues. J Therm Anal Calorim. 2017;130:2301–14.
Liu G-X, Xu Y-Y, Ren X-M, Nishihara S, Huang R-Y. Self-assembly of 3-D 4d–4f coordination frameworks based on pyridine-3,5-dicarboxylic acid: syntheses, crystal structures and luminescence. Inorg Chim Acta. 2010;363:3727–32.
Acknowledgements
RD is thankful to the University of Kerala, Trivandrum, India, for the University Research Fellowship. We sincerely thank Dr. R. Rajeev, Head, Analytical and Spectroscopy Division, VSSC, Trivandrum, Kerala, for his help and valuable suggestions on thermal decomposition studies. We are also grateful to Dr. Shibu M. Eapen, SAIF, CUSAT, Kochi, India, for single-crystal X-ray diffraction measurements and Dr. A. Santhosh Kumar, School of Pure and Applied Physics, M. G University, Kottayam, India, for luminescent studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drisya, R., Soumyamol, U.S., Satheesh Chandran, P.R. et al. Structural, thermal decomposition and luminescent studies on gel grown crystals of poly[tetraaquadinicotinatostrontium(II)] containing ‘χ’-shaped hydrophobic channels. J Therm Anal Calorim 134, 1987–1999 (2018). https://doi.org/10.1007/s10973-018-7868-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7868-5