Abstract
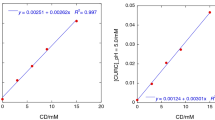
The effect of solution pH on the formation of an inclusion complex between (−)-epigallocatechin gallate (EGCg: pKa = 7.5) and β-cyclodextrin (β-CD) was investigated by isothermal titration calorimetry and 1H-NMR spectroscopy. The formation of an inclusion complex (EGCg-β-CD) depended on the solution pH; two different types of inclusion complexes were formed at 1:1 molar ratio in acid/neutral solutions, and only one type of complex was formed in the basic solution. The first type of EGCg-β-CD with larger association constant was formed independently of pH, with the AC-ring of EGCg being deeply inserted into the cavity of β-CD and the B-ring existing near the secondary hydroxyl group of β-CD. On the other hand, the formation of the second type depended on the solution pH. The B′-ring of EGCg was included in the case of acid and neutral solutions, but the formation of an inclusion complex in the basic solution was difficult due to the ionization of the 4″-OH on the B′-ring. 1H-NMR spectroscopy supported these results. These results suggested that when determining the structures of EGCg-β-CD in an aqueous solution, it is necessary to consider unionized and ionized forms of EGCg.




Similar content being viewed by others
References
Ikeda H, Moriwaki H, Matsubara T, Yukawa M, Iwase Y, Yukawa E, Aki H. Mechanism of interaction between risperidone and tea catechin (2) influence of presence of galloyl group in catechin on insoluble complex formation with risperidone. Yakugaku Zasshi. 2012;132:145–53.
Inoue MB, Inoue M, Fernando Q, Valcic S, Timmermann BN. Potentiometric and 1H NMR studies of complexation of Al3+ with (−)-epigallocatechin gallate, a major active constituent of green tea. J Inorg Biochem. 2002;88:7–13.
Okumura H, Ichitani M, Takihara T, Kunimoto K. Facile quantitative analysis of gallated catechins in tea beverage by UV absorption spectra. Jpn J Food Chem. 2007;14:128–33.
Ohata T, Ikeda H, Inenaga M, Mizobe T, Yukawa M, Fujisawa M, Aki H. Drug-tea polyphenol interaction (II) complexation of piperazine derivatives with green tea polyphenol. Thermochim Acta. 2017;653:1–7.
Ikeda H, Tsuji E, Matsubara T, Yukawa M, Fujisawa M, Yukawa E, Aki H. Incompatibility between propericiazine oral solution and tea-based drink. Chem Pharm Bull. 2012;60:1207–11.
Yokozawa T, Cho EJ, Hara Y, Kitani K. Antioxidative activity of green tea treated with radical initiator 2,2′-azobis (2-amidinopropane) dihydrochloride. J Agric Food Chem. 2000;48:5068–73.
Lee LS, Kim SH, Kim YB, Kim YC. Quantitative analysis of major constituents in green tea with different plucking periods and their antioxidant activity. Molecules. 2014;19:9173–86.
Kobayashi M, Nishizawa M, Inoue N, Hosoya T, Yoshida M, Ukawa Y, Sagesaka YM, Doi T, Nakayama T, Kumazawa S, Ikeda I. Epigallocatechin gallate decreases the micellar solubility of cholesterol via specific interaction with phosphatidylcholine. J Agric Food Chem. 2014;62:2881–90.
Asahi Y, Noiri Y, Miura J, Maezono H, Yamaguchi M, Yamamoto R, Azakami H, Hayashi M, Ebisu S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J Appl Microbiol. 2014;116:1164–71.
Tamura M, Saito H, Kikuchi K, Ishigami T, Toyama Y, Takami M, Ochiai K. Antimicrobial activity of gel-entrapped catechins toward oral microorganisms. Biol Pharm Bull. 2011;34:638–43.
Khan N, Mukhtar H. Tea and health: studies in humans. Curr Pharm Des. 2013;19:6141–7.
Yukawa M, Moriwaki H, Murakami T, Ikeda H, Iwase Y, Aki H. Effect of pH on the conformation of inclusion complexes between β-lactam antibiotics and β-cyclodextrins in aqueous solution. Kobunshi Ronbunshu. 2010;67:192–7.
Wszelaka-Rylik M. Thermodynamics of β-cyclodextrin–ephedrine inclusion complex formation and covering of nanometric calcite with these substances. J Therm Anal Calorim. 2017;127:1825–34.
Ikeda H, Fukushige Y, Matsubara T, Inenaga M, Kawahara M, Yukawa M, Fujisawa M, Yukawa E, Aki H. Improving water solubility of nateglinide by complexation of β-cyclodextrin. J Therm Anal Calorim. 2016;123:1847–50.
Sbârcea L, Udrescu L, Ledeţi I, Szabadai Z, Fuliaş A, Sbârcea C. β-Cyclodextrin inclusion complexes of lisinopril and zofenopril. J Therm Anal Calorim. 2016;123:2377–90.
Semalty A, Tanwar YS, Semalty M. Preparation and characterization of cyclodextrin inclusion complex of naringenin and critical comparison with phospholipid complexation for improving solubility and dissolution. J Therm Anal Calorim. 2014;115:2471–8.
Ishizu T, Hirata C, Yamamoto H, Harano K. Structure and intramolecular flexibility of β-cyclodextrin complex with (−)-epigallocatechin gallate in aqueous solvent. Magn Reson Chem. 2006;44:776–83.
Aki H, Niiya T, Iwase Y, Kawasaki Y, Kumai K, Kimura T. Multimodal inclusion complexes of ampicillin with β-cyclodextrins in aqueous solution. Thermochim Acta. 2004;416:87–92.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ohata, T., Ikeda, H., Mizobe, T. et al. Effect of solution pH on complex formation between epi-type catechin and β-cyclodextrin. J Therm Anal Calorim 135, 2837–2841 (2019). https://doi.org/10.1007/s10973-018-7602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7602-3