Abstract
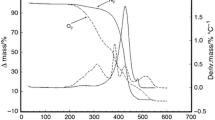
Thermal profiles of buriti pulp oil (Mauritia flexuosa Mart.), tucumã pulp and kernel oils (Astrocarium vulgare Mart.), rubber seed oil (Hevea brasiliensis), passion fruit oil (Passiflora edulis) and ucuúba butter (Virola surinamensis) were analyzed by thermogravimetry (TG/DTG) and differential scanning calorimetry (DSC). Gas chromatography and calculated iodine values were performed to determine the fatty acid profile and to measure the degree of unsaturation in these oils, respectively. The TG curves showed three steps of mass loss, which can be attributed to the degradation of polyunsaturated, monounsaturated and saturated fatty acids. The DSC crystallization and melting curves are reported and depended on the fatty acid composition. Usually, oil samples with a high degree of saturation showed crystallization and melting profiles at higher temperatures than the oils with a high degree of unsaturation. The data obtained by physicochemical analysis of oil samples were analyzed by principal component analysis and hierarchical cluster analysis to increase understanding of the data set, examining the presence or absence of natural groupings between samples.






Similar content being viewed by others
References
Pardauil JJR, Souza LKC, Molfetta FA, Zamian JR, Rocha Filho GN, Costa CEF. Determination of the oxidative stability by DSC of vegetable oils from the Amazonian area. Bioresour Technol. 2011;102:5873–7.
Santos OV, Corrêa NCF, Carvalho RN Jr, Costa CEF, França LFF, Lannes SCS. Comparative parameters of the nutritional contribution and functional claims of Brazil nut kernels, oil and defatted cake. Food Res Int. 2013;51:841–7.
Cunha MAE, Neves RF, Souza JNS, França LF, Araújo ME, Brunner G, Machado NT. Supercritical adsorption of buriti oil (Mauritia flexuosa Mart.) in—alumina: a methodology for the enriching of anti-oxidants. J Supercrit Fluids. 2012;66:181–91.
Forero-Doria O, Gallego J, Valdes O, Pinzon-Topa C, Santos LS, Guzma L. Relationship between oxidative stability and antioxidant activity of oil extracted from the peel of Mauritia flexuosa fruits. J Therm Anal Calorim. 2016;123:2173–8.
Lima JRO, Silva RB, Moura EM, Moura CVR. Biodiesel of tucum oil, synthesized by methanolic and ethanolic routes. Fuel. 2008;87:1718–23.
Lira CS, Berruti FM, Palmisano P, Berruti F, Briens C, Pécora AAB. Fast pyrolysis of Amazon tucumã (Astrocaryum aculeatum) seeds in a bubbling fluidized bed reactor. J Anal Appl Pyrol. 2013;99:23–31.
Souza Filho OC, Sagrillo MR, Garcia LFM, Machado AK, Cadona F, Ribeiro EE, Duarte MMMF, Morel AF, Cruz IBM. The in vitro genotoxic effect of Tucuma (Astrocaryum aculeatum), an Amazonian Fruit Rich in Carotenoids. J Med Food. 2013;16:1013–21.
Salimon J, Abdullah BM, Salih N. Rubber (Hevea brasiliensis) seed oil toxicity effect and Linamarin compound analysis. Lipids Health Dis. 2012;11:74.
Silva JK, Cazarin CBB, Colomeu TC, Batista AG, Meletti LMM, Paschoal JAR, Bogusz S Jr, Furlan MF, Reyes FG, Maróstica MR Jr, Zollner RL. Antioxidant activity of aqueous extract of passion fruit (Passiflora edulis) leaves: in vitro and in vivo study. Food Res Int. 2013;53:882–90.
Stecanella LA, Taveira SF, Marreto RN, Valadares MC, Vieira MS, Kato MJ, Lima EM. Development and characterization of PLGA nanocapsules of grandisin isolated from Virola surinamensis: in vitro release and cytotoxicity studies. Braz J Pharmacogn. 2013;23:153–9.
Samyn P, Schoukens G, Vonck L, Stanssens D, Van den Abbeele H. Quality of Brazilian vegetable oils evaluated by (modulated) differential scanning calorimetry. J Therm Anal Calorim. 2012;110:1353–65.
Sessa DJ. Derivation of a cocoa butter equivalent from Jojoba transesterified ester via a differential scanning calorimetry index. J Sci Food Agric. 1996;72:295–8.
Calligaris S, Arrighetti G, Barba L, Nicoli MC. Phase transition of sunflower oil as affected by the oxidation level. J Am Oil Chem Soc. 2008;85:591–8.
Tan CP, Che Man YB. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc. 2000;77:143–55.
American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 4th ed. Champaign, USA (A.O.C.S.Official Method Ce 1-62: Fatty acid composition by gas chromatography); 1995.
American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 4th ed. Champaign, USA (A.O.C.S.Official Method Ce 2-66: Preparation of methyl esters of long chain fatty acids); 1995.
American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 5th ed. Champaign, USA (A.O.C.S.Official Method Cd 3d-63: Acid value); 1999.
American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 4th ed. Champaign, USA (A.O.C.S.Official Method Cd 8-53: Peroxide value); 1997.
American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 4th ed. Champaign, USA (A.O.C.S.Official Method AOCS Tl 1a-64: Saponification Value); 1997.
American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 5th ed. Champaign, USA (A.O.C.S.Official Method AOCS Cd 1c-85: Calculated Iodine Value); 1997.
Molfetta FA, Bruni AT, Honório KM, Silva ABF. A structure–activity relationship study of quinone compounds with trypanocidal activity. Eur J Med Chem. 2005;40:329–38.
Anihouvi PP, Blecker C, Dombree A, Danthine S. Comparative study of thermal and structural behavior of four industrial lauric fats. Food Bioprocess Technol. 2013;6:3381–91.
Regitano-d’Arce MAB. Química básica dos lipídeos. In: Oetterer M, Regitano-d’Arce MAB, Spoto MHF, editors. Fundamentos de ciência e tecnologia de alimentos. Manole, Barueri-SP; 2006. p. 196–242.
BRASIL. Resolução RDC/ANVISA/MS nº 270, de 22 de setembro de 2005. Regulamento técnico para óleos vegetais, gorduras vegetais e creme vegetal. Diário Oficial da República Federativa do Brasil. Brasília, DF, 23 setembro 2005. Seção 1.
Lopes NP, Kato M, Yoshida M. Antifungal constituents from roots of Virola surinamensis. Phytochemistry. 1999;51:29–33.
Costa MNFS, Muniz MAP, Negrão CAB, Costa CEF, Lamarão MLN, Morais L, Silva Jr JOC, Costa RMR. Characterization of Pentaclethra macroloba oil: thermal stability, gas chromatography and Rancimat. J Therm Anal Calorim. 2014;115:2269–75.
Santos JCO, Santos IMG, Souza AG, Prasad S, Santos AV. Thermal stability and kinetic study on thermal decomposition of commercial edible oils by thermogravimetry. J Food Sci. 2002;67:1393–8.
Dweck J, Sampaio CMS. Analysis of the thermal composition of commercial vegetable oils in air by simultaneous TG/DTA. J Therm Anal Calorim. 2004;75:419–28.
Santos AGD, Caldeira VPS, Souza LD, Oliveira DS, Araujo AS, Luz GE Jr. Study of the thermal stability by thermogravimetry for oil, biodiesel and blend (B10) of different oilseeds. J Therm Anal Calorim. 2016;123:2021–8.
Garcia CC, Franco PIBM, Zuppa TO, Antoniosi Filho NR, Leles MIG. Thermal stability studies of some cerrado plant oils. J Therm Anal Calorim. 2007;87:645–8.
Vecchio E, Cerretani L, Bendini A, Chiavaro E. Thermal decomposition study of monovarietal extra virgin olive oil by simultaneous thermogravimmetry/differential scanning calorimetry: relation with chemical composition. J Agric Food Chem. 2009;57:4793–800.
Tan CP, Che Man YB. Differential scanning calorimetric analysis of palm oil. Palm oil based products and coconut oil: effects of scanning rate variation. Food Chem. 2002;76:89–102.
Kobelnik M, Fontanari GG, Soares RAM, Figueiredo AG, Ribeiro CA. Study of the thermal behavior of bicuı’ba oil (Virola bicuhyba). J Therm Anal Calorim. 2014;115:2107–13.
Tengku-Rozaina TM, Birch EJ. Effects of fractionation on melting and crystallisation profiles of hoki oil measured by DSC. J Therm Anal Calorim. 2015;120:395–402.
Tolstorebrov I, Eikevik TM, Bantle M. A DSC determination of phase transitions and liquid fraction in fish oils and mixtures of triacylglicerides. Food Res Int. 2014;58:132–40.
Acknowledgements
The authors would like to thank CAPES Foundation, Ministry of Education of Brazil, for a Doctoral scholarship to Pardauil, J. J. R, and FINEP/SIPI, FAPESPA/VALE and CNPq for financial support. Also, the authors would like to thanks to Amazon Oil Industry for providing the oils used in this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pardauil, J.J.R., de Molfetta, F.A., Braga, M. et al. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J Therm Anal Calorim 127, 1221–1229 (2017). https://doi.org/10.1007/s10973-016-5605-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5605-5