Abstract
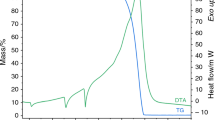
The ammonium, barium hexacyanoferrate(II) trihydrate, Ba(NH4)2[Fe(CN)6]·3H2O, has been synthesized for the first time, was characterized by thermogravimetric and differential thermal analysis (TG–DTA), infrared (IR) and Raman spectroscopy and chemical analysis, and its crystal and molecular structures were determined by X-ray diffraction methods. The chemical composition was determined by assaying Ba(II) with EDTA, ammonia nitrogen with Nessler’s reagent as indicator and Fe(II) using spectrophotometry with ortho-phenanthroline method. The hydration number was estimated by thermogravimetric analysis. The compound crystallizes in the trigonal R-3c space group. The ferrocyanide anion has an almost perfect octahedral shape with its Fe(II) ion in a crystallographic special position of point symmetry S6 [d(Fe–C) = 1.912(2) Å, d(C–N) = 1.153(3) Å]. The barium, ammonium nitrogen and water oxygen atoms are also at lattice special positions with site symmetries D3, C3 and C2, respectively. The thermal decomposition process was also studied using TG–DTA, and the products of decomposition were identified by IR spectroscopy. It proposes a mechanism of decomposition.




Similar content being viewed by others
References
Wu L, Yu J, Zhang L, Wang X, Li S. Selective self-propagating combustion synthesis of hexagonal and orthorhombic nanocrystalline yttrium iron oxide. J Solid State Chem. 2004;177:3666–74.
Lu X, Xie J, Shu H, Liu J, Yin C, Lin J. Microwave-assisted synthesis of nanocrystalline YFeO3 and study of its photoactivity. Mater Sci Eng B. 2007;138:289–92.
Lentmaier J, Kemmier-Sack S, Knell G, Kessier P, Plies P. Selective reduction of nitrogen monoxide by catalysts based on composites between solid acid and perovskite in the presence of excess oxygen. Mater Res Bull. 1996;31:1269–76.
Lentmaier J, Kemmier-Sack S. Bifunctional YFeO3-based catalysts used in the selective catalytic reduction of nitrogen monoxide in the presence of excess oxygen. Mater Res Bull. 1998;33:461–73.
Scott MJ, Holm RH. Molecular assemblies containing linear and Bent [FeIII-CN-CuII] bridge units: synthesis, structure, and relevance to cyanide-inhibited heme-copper oxidases. J Am Chem Soc. 1994;116:11357–67.
Iwamoto T. Supramolecular chemistry in cyanometallate systems. In: Lehn JM. Comprehensive supramolecular chemistry. London: Pergamon; 1996.
Knoeppel DW, Shore SG. Cyanide-bridged lanthanide–transition metal one-dimensional arrays {(DMF)10Yb2[Ni(CN)4]3}∞ and {(DMF)10Yb2[Pt(CN)4]3}∞. Inorg Chem. 1996;35:1747–8.
Zhang HX, Tong YX, Chen ZN, Yu KB, Kang BS. Cyano-bridged extended heteronuclear supramolecular architectures with hexacyanoferrates(II) as building blocks. J Organomet Chem. 2000;598:63–70.
Černák J, Orendáč M, Potočňák I, Chomič J, Orendáčová A, Skoršepa J, Feher A. Cyanocomplexes with one-dimensional structures: preparations, crystal structures and magnetic properties. Coord Chem Rev. 2002;224:51–66.
Nakayama S, Sakamoto M, Matsuki K, Okimura Y, Ohsumi R, Nakayama Y, Sadaoka Y. Preparation of perovskite-type LaFeO3 by thermal decomposition of heteronuclear complex, {La[Fe(CN)6]·5H2O}x. Chem Lett. 1992;11:2145–7.
Matsuura Y, Matsushima S, Sakamoto M, Sadaoka Y. NO2-sensitive LaFeO3 film prepared by thermal decomposition of the heteronuclear complex, {La[Fe(CN)6]·5H2O}x. J Matter Chem. 1993;3:767–9.
Sadaoka Y, Watanabe K, Sakai Y, Sakamoto M. Preparation of perovskite-type oxides by thermal decomposition of heteronuclear complexes, {Ln[Fe(CN)6]·nH2O}x, (Ln–La ~ Ho). J Alloys Compd. 1995;224:194–8.
Kondo N, Itoh H, Kurihara M, Sakamoto M, Aono H, Sadaoka Y. New high-yield preparation procedure of Ln[Fe(CN)6]·nH2O (Ln=La, Gd and Lu) and their thermal decomposition into perovskite-type oxides. J Alloys Compd. 2006;408:1026–9.
Gómez MI, de Morán JA, Carbonio RE, Aymonino PJ. Synthesis of AFeO2.5+x (0 ≤ x ≤ 0,5; A = Sr, Ca) mixed oxides from the oxidative thermal decomposition of A[Fe(CN)5NO]·4H2O. J Solid State Chem. 1999;142:138–45.
Gómez MI, de Morán JA, Aymonino PJ, Pagola S, Stephens P, Carbonio RE. Ab initio structure solution of BaFeO2.8−δ, a new polytype in the system of BaFeOy (2.5 ≤ y≤3.0) prepared from the oxidative thermal decomposition of BaFe[(CN)5NO]·3H2O. J Solid State Chem. 2001;160:17–24.
Navarro MC, Pannunzio-Miner EV, Pagola S, Gómez MI, Carbonio RE. Structural refinement of Nd[Fe(CN)6]·4H2O and study of NdFeO3 obtained by its oxidative thermal decomposition at very low temperatures. J Solid State Chem. 2005;178:847–54.
Córdoba LM, Gómez MI, de Morán JA, Aymonino PJ. Synthesis of the SrFeO2.5 and BaFeO3−x perovskites by thermal decomposition of SrNH4[Fe(CN)6]·3H2O and BaNH4[Fe(CN)6]. J Argent Chem Soc. 2008;96:1–2.
Gil DM, Navarro MC, Lagarrigue MC, Guimpel J, Carbonio RE, Gómez MI. Synthesis and structural characterization of perovskite YFeO3 by thermal decomposition of a cyano complex precursor, Y[Fe(CN)6]·4H2O. J Therm Anal Calorim. 2011;103:889–96.
Gil DM, Guimpel J, Paesano A Jr, Carbonio RE, Gómez MI. Y[Fe1−xCox(CN)6]·4H2O (0 ≤ x ≤ 1) solid solutions: synthesis, crystal structure, thermal decomposition and spetroscopic and magnetic properties. J Mol Struct. 2012;1015:112–7.
Gil DM, Navarro MC, Lagarrigue MC, Guimpel J, Carbonio RE, Gómez MI. Crystal structure refinement, spectroscopic study and magnetic properties of yttrium hexacyanoferrate(III). J Mol Struct. 2011;1003:129–33.
Kumar Swamy N, Pavan Kumar N, Venugopal Reddy P, Manish Gupta, Shanmukharao Samatham S, Venkateshwarulu D, Ganesan V, Vikas Malik, Das BK. Specific heat and magnetocaloric effect studies in multiferroic YMnO3. J Therm Anal Calorim. 2014. doi:10.1007/s10973-014-4223-3.
Bailey WE, Williams RJ, Milligan WO. The crystal structure of La[Fe(CN)6]·5H2O. Acta Crystallogr B. 1973;29:1365–8.
Kietaibl H, Petter W. Die Kristallstrukturen von La[Fe(CN)6]·5H2O und Sm[Fe(CN)6]·4H2O. Helv Phys Acta. 1974;47:425.
Bonnet MC, Paris RA. Hexacyanometallates: part 1: radiographic and infrared study of lanthanide hexacyanoferrates(III). Bull Soc Chim Fr. 1975;5–6:1062–6.
Bonnet MC, Paris RA. Lanthanide hexacyanometallates: part 2: radiocrystallographic and infrared study of lanthanide hexacyanocobaltates(III) thermal decomposition. Bull Soc Chim Fr. 1975;5–6:1067–70.
Navarro MC, Lagarrigue MC, De Paoli JM, Carbonio RE, Gómez MI. A new method of synthesis of BiFeO3 prepared by thermal decomposition of Bi[Fe(CN)6]·4H2O. J Therm Anal Calorim. 2010;102:655–60.
Navarro MC, Lagarrigue MC, Carbonio RE, Gómez MI. Synthesis, characterization and study of products of the thermal decomposition of Bi[FexCo1−x(CN)6]·4H2O. Glob J Inorg Chem. 2010;1:76–82.
Gil DM, Ávila M, Reguera E, Pagola S, Gómez MI, Carbonio RE. Lead hexacyanoferrate(II) tetrahydrate: crystal structure, FTIR spectroscopy and thermal decomposition studies. Polyhedron. 2012;33:450–5.
Raistrick ID, Endow N, Lewkowitz S, Huggins RA. Structural aspects of some mixed metal ferrocyanides. J Inorg Nucl Chem. 1977;39:1779–83.
Medina Córdoba L, de Morán JA, Santos S Jr, Piro OE, Gómez MI. Synthesis and crystal structure of ammonium, strontium hexacyanoferrate(II) dihydrate. J Chem Crystallogr. 2011;41:1280–6.
Gallagher PK. A simple technique for the preparation of R.E. FeO3 and R.E. CoO3. Mater Res Bull. 1968;3:225–32.
Traversa E, Nunziante P, Sakamoto M, Sadaoka Y, Carotta MC, Martinelli G. Thermal evolution of the microstructure of nanosized LaFeO3 powders from the thermal decomposition of a heteronuclear complex, La[Fe(CN)6]·5H2O. J Mater Res. 1998;13:1335–43.
Gil DM, Carbonio RE, Gómez MI. Synthesis of Pb2Fe2O5 by thermal decomposition of Pb2[Fe(CN)6]·4H2O. J Chil Chem Soc. 2010;55(2):189–92.
Navarro MC, Lagarrigue MC, De Paoli JM, Carbonio RE, Gómez MI. Synthesis and structural characterization of perovskite YFeO3 by thermal decomposition of a cyano complex precursor Y[Fe(CN)6]·4H2O. J Therm Anal Calorim. 2010;102:655–60.
Williams HE. Cyanogen compounds. London: Arnold; 1948.
Ayres GH. Análisis químico cuantitativo. Spain: Harper & Row Inc; 1991.
Rodier J. Análisis de las aguas. Barcelona: Omega SA; 1978.
Vogel I. Química analítica cuantitativa. Argentina: Kapelusz; 1969.
CrysAlisPro, Oxford Diffraction Ltd., version 1.171.33.48 (release 15-09-2009 CrysAlis171.NET).
Sheldrick GM. A short history of SHELX. Acta Crystallogr A. 2008;64:112–22.
Hurlbut CS, Klein C Jr. Manual de minerología. 4ª ed. España: Reverté SA; 1997.
Johnson CK. ORTEP-II: a Fortran Thermal-Ellipsoid Plot Program. Report ORNL-5318, Oak Ridge National Laboratory, Tennessee, USA, 1976.
Lide DR. Handbook of chemistry and physics. 80th Ed. New York: Chemical Rubber Publishing Company; 1999–2000.
Deb N. The solid state thermal decomposition of Sr3[La(C2O4)3(H2O)2]2·11H2O. J Therm Anal Calorim. 2012;114(1):261–7.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. New York: Wiley; 1986.
Gil DM, Carbonio RE, Gómez MI. Crystal structure refinement and vibrational analysis of Y[Co(CN)6]·4H2O and its thermal decomposition products. J Mol Struct. 2013;104:23–8.
Park S-K, Lee C-K, Lee S-H, Lee N-S. Vibrational analysis of ferrocyanide complex ion based on density functional force field. Bull Korean Chem Soc. 2002;23(2):253–61.
Acknowledgements
This work was supported by CONICET (PIP 1529), and by ANPCyT (PME06 2804 and PICT06 2315) of Argentina. G.A.E. and O.E.P are Research Fellows of CONICET. L.M.C. and M.I.G. thank Consejo de Investigaciones de la Universidad Nacional de Tucumán (CIUNT) for financial support, Project 26D-517.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Córdoba, L.M., Echeverría, G.A., Piro, O.E. et al. Ammonium, barium hexacyanoferrate(II) trihydrate. J Therm Anal Calorim 120, 1827–1834 (2015). https://doi.org/10.1007/s10973-015-4492-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4492-5