Abstract
Highly porous nanocomposite films were produced at room temperature from SiO2 or TiO2–SiO2 solutions synthesized by a sol–gel route in the presence of oxide nanoparticles. The photocatalytic behavior of titania-based films was controlled at the sol synthesis step, through three parameters: the relative molar concentration between polymeric and condensed silica, the reactivity of the hydrolysis step, and the relative molar concentration of the active component. The reported method allowed ample tuneability of film refractive index, an important parameter for the design of antireflective and multifunctional coatings. Superhydrophilic properties were demonstrated by the samples and, notably, retained for over 30 days. Experimentally mimicking accelerated surface pollution with olive oil and stearic acid, a strong correlation was found between residual organic contamination, quantified by integrating the film absorption FT-IR spectrum over the C–Hn stretching area, and the measured water contact angle (WCA). Strategies for a fast surface cleaning and restoration of the superhydrophilic behavior via a mild oxidizing agent, based on UV exposure or immersion in a NaClO water solution, were investigated.

High wettability can also be obtained through the control of the porosity. This effect has been termed nanowicking: the rapid infiltration of water in an interconnected porous network.
Highlights
-
Superhydrophilic highly porous nanocomposite films are realized through the effect of nanowicking.
-
Films are produced by sol–gel at room temperature from SiO2 or TiO2–SiO2 in the presence of oxide nanoparticles.
-
Ample tuneability of refractive index is achieved, allowing the design of antireflective and multifunctional coatings.
-
Superhydrophilic properties are retained by the samples over 30 days.
-
Fast surface cleaning and superhydrophilicity restoration is demonstrated UV exposure or immersion in a NaClO water solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fog formation on surfaces is a major challenge in several optical systems, such as the lenses and mirrors found in glasses, swimming goggles, camera lenses, binoculars, etc. This undesirable phenomenon reduces the effectiveness of light transmittance and therefore the optical surface and material efficiency [1].
The development of anti-fog coatings deals with the creation of hydrophilic surfaces, such that water naturally forms a continuous, or nearly continuous, film rather than dispersed droplets on them, therefore it is related to an optimization of the parameters that control the wetting behavior of a surface: surface energy, which depends on its chemical composition, and surface roughness/texture, which can enhance both hydrophilicity and hydrophobicity [2,3,4,5]. Complete wetting occurs when a surface is readily invaded by water and can accommodate the full volume of a liquid droplet within its surface texture. This phenomenon is realized in the presence of a so-called superhydrophilic surface, which is defined as a surface whose water contact angle (WCA) becomes 5° within 0.5 s or less [6].
Materials presenting high surface energy are ideal candidates for the development of anti-fog surfaces. Among them, silicon dioxide and titanium dioxide are preferred due to the availability of starting materials in the form of nanoparticles. While the starting WCA is similar on both oxides, silica (SiO2) surfaces show a lower contamination rate and, hence, a slower increase with time in WCA when compared to titania (TiO2)–based samples, which results from the higher TiO2 surface energy. However, the photocatalytic properties and UV-activated superhydrophilicity of TiO2 [7] allow for a fast recovery of the low WCA after exposure, thus providing a simple and reliable method to maintain anti-fog properties over time [8]. For the engineering of anti-fog coatings based on TiO2 nanoparticles, the proper crystalline form, the high refractive index, and the high absorption coefficient should be taken into account, as these characteristics necessarily limit the range of concentrations of TiO2 on formulations to be deposited by coating methods. The use of SiO2–TiO2 mixed oxides have been shown to be a promising way to combine self-cleaning properties with low refractive indices and with a porosity control, in order to enhance superhydrophilicity and long-term stability [9]. Nonetheless, an anti-fog behavior can be restored on contaminated SiO2 surfaces by the use of mild oxidizing solutions.
Together with surface energy, surface roughness determines the apparent WCA, so that hydrophilic or hydrophobic characters are enhanced. For nanocomposite systems based on oxide nanoparticles, a proper choice of particle size, binder amount, and condensation mechanism are reflected by surface roughness. In superhydrophilic coatings, the role of porosity is to increase the surface available to water interaction as well as to control the final film optical properties, helping to lower the refractive index in the presence of TiO2 nanoparticles.
The creation of a nanoporous thin-film coating with a low refractive index also promotes an antireflection behavior. The optical thickness of the coating (product of refractive index and thickness) can be tuned by simply varying the assembly conditions and the number of deposited material layers. In order to obtain multifunctional coatings that combine anti-fogging with antireflection capabilities, it is necessary to achieve the critical thickness needed for a super wetting behavior and the optical thickness required for maximum suppression of reflections over the desired wavelength range.
Several routes are possible for anti-fog coating application on a material surface: physical or chemical vapor deposition, layer-by-layer assembly, electrochemical methods, etc. [10]. However, while these techniques have demonstrated their usefulness and effectiveness, they are expensive and cumbersome. Furthermore, the range and shape of materials that can be coated by these techniques are restricted.
An alternative strategy involves the use of sol−gel processing of metal alkoxides or organically-modified precursors for the synthesis of liquid solutions, that can be readily deposited by dip coating or spin coating techniques on different substrates at (or near) room temperature [11,12,13,14]. A sol–gel process requires low-cost equipment and facile processing steps, significantly simplifying the production of functional coatings on optical surfaces. In this work, all silica and silica/titania nanocomposite coatings were produced through this method, enabling control over film composition, refractive index, wetting properties, and ultimately anti-fog character. The distinctiveness of the reported nanocomposite coatings is the enhanced durability, with respect to analog functional coatings reported in the literature [9, 15, 16], which was experimentally demonstrated by reproducing controlled conditions for accelerated pollution of the samples. Strategies for a fast surface cleaning via a mild oxidizing agent, based on UV exposure or wetting restoring, are proposed.
2 Experimental
2.1 Synthesis and deposition
2.1.1 Preparation of TiO2–SiO2 solutions
TiO2–SiO2 solutions were prepared from silica sols synthesized by a sol–gel route with the addition of silica or titania colloids. Silica nanoparticles of 12 or 22 nm diameter in H2O at a concentration of 30 or 50 wt%, respectively called LS and TMA, were purchased from Grace Davison. Titania anatase nanoparticles of 15 nm diameter in 2-Propanol (IPA) at 15 wt% concentration were purchased from MKNano (M K Impex Corp.). A SiO2 polymeric sol was prepared by diluting tetraethoxysilane (TEOS, Aldrich) in absolute ethanol, deionized water, and hydrochloric acid (HCl). Concentrated sols (mother solutions, MS) were prepared with a TEOS concentration of 2.4 M, an H2O/TEOS molar ratio varying in the 2–14 range, and a pH from 1 to 4, successively adding the desired amount of SiO2 nanoparticles. When SiO2/TiO2 nanoparticles were both added, MS was prepared to start from the hydrolysis of TEOS in an acidic environment in the presence of SiO2 nanoparticles. Once hydrolysis was completed, TiO2 nanoparticles were added to be entrapped within the silica network. Given the different charges of SiO2 and TiO2 at the same acidic pH, the mixture of the two sols was possible only subsequently to a hydrolysis step, after which SiO2 nanoparticles were reasonably covered in entangled silica polymers.
The photocatalytic behavior of the final films was controlled at the sol synthesis step, through three different parameters: the relative molar concentration between polymeric and condensed silica: [TEOS]/[SiO2 nanoparticles]; the reactivity of the hydrolysis step: Rw = [H2O]/[TEOS]; and the relative molar concentration of active component: [TiO2]/([SiO2]+[TiO2]).
The prepared solutions were stable for months, as evidenced by reproducible film depositions.
2.1.2 Synthesis details
In the presence of water, ethoxy groups of the TEOS precursor are partially hydrolyzed according to the simplified reaction:
where R is an ethyl group, and thus OR an ethoxy group and ROH ethanol. This reaction describes an equilibrium between the hydrolysis of ethoxy groups and the reverse reaction (re-esterification). Then, the hydrolyzed groups can react by condensation to form Si–O–Si bridging bonds according to the following reactions:
Reaction (2) prevails for sols with high water content (Rw ≥ 2) while reaction (3) prevails for water-deficient sols (Rw«2). In both cases, it is the content of Si–OH silanol groups formed in reaction (1) which induces the condensation reactions. Reactions (1) and (2)–(3) proceed simultaneously (polycondensation) and lead to the creation of oligomeric chains –(O–Si)n– diluted in the solvent. During solvent evaporation, polycondensation of these chains takes place, yielding a silica polymeric network with non-hydrolyzed Si–OR or silanol Si–OH chain-end groups. The chain structure, length, and cross-linking degree for this polymeric network are significantly influenced by the concentration C of the TEOS precursor (C = [TEOS]), molar ratio Rw, and pH. In particular, C determines the probability of reactive species diluted in ethanol to contact each other and react by polycondensation. C and Rw determine the equilibrium between hydrolysis and re-esterification and, as a consequence, the content of silanol groups that may react in reactions (2)–(3). Furthermore, since hydrolysis and condensation reactions require activation of Si–OH bonds under acidic conditions, they are promoted under highly acidic conditions due to the significant proportion of H3O+ (hydrolysis) and Si–OH2+ end-chain (condensation) species formed in the acidic sol. On the contrary, the kinetics of condensation decreases as the pH approaches the isoelectric point of silica (IEP = 2) as nearly neutral species formed at such pH conditions are much less prone to a nucleophilic attack.
2.1.3 Deposition
The sols were spin-coated over soda-lime glass substrates or silicon wafers at the desired rpm for 120 s. The samples were subsequently thermally treated in the oven at 80 °C for 30 min to promote silica network stabilization.
2.2 Characterization
2.2.1 FT-IR spectroscopy
In order to study the extent of reaction of sol–gel systems and the microstructure of silica films, infrared absorption spectra were recorded in the range of 4000–400 cm−1 range by a Fourier Transform Infrared Spectrometer (Jasco FT/IR-620) with a resolution of 4 cm−1, averaging over 32 scans.
One of the main issues was to resolve the contribution of condensed silica of LS and TMA nanoparticles, from peaks corresponding to unreacted, hydrolyzed, or condensed silica species from TEOS sol–gel processing.
FT-IR absorption spectra of silica thin layers are characterized by three main absorption bands assigned to different vibrational modes of the Si–O–Si bonds. [17]. The lowest frequency mode peaked at 460 cm−1 is assigned to transverse-optical TO1 rocking motions of the oxygen atoms bridging two adjacent Si atoms, perpendicular to the Si–O–Si plane. TO2 symmetric stretching of the O atom along a line bisecting the Si–O–Si angle is observed near 800 cm−1. The higher wavenumber mode at 1070 cm−1 involves the motion of the O atom along a line parallel to the Si–Si axis; the motion results in opposite distortion of two neighboring Si–O bonds and is indicated as TO3 antisymmetric. This band is always accompanied by an intense shoulder at the higher wavenumber side. All three TO vibrational modes show longitudinal-optic (LO)-TO splitting [18] to different extents: the rocking mode LO1–TO1, at 507–457 cm−1, the symmetric stretching LO2–TO2 at 820–810 cm−1, and the antisymmetric stretching LO3–TO3, at 1250–1070 cm−1. LO1,2 are not observed in FT-IR spectra, while LO3 is assigned to the 1250 cm−1 shoulder of the TO3 vibration band. Even if the LO3 mode is not detected in transmission measurements at normal incidence (as only TO modes are excited), the shoulder is a signature of the LO component of the TO3 antisymmetric stretching or a mixed LO–TO mode. The observation of this shoulder has particular importance as it is much enhanced in highly porous silica systems. The intensity of this mode is observed to increase with porosity in thin films because of the scattering of the IR radiation within the pores and the consequent activation of the LO modes for oblique incidence [19,20,21].
2.2.2 Ellipsometry
The porosity and composition of the produced nanoparticle multilayer coatings were studied by ellipsometry.
Thickness and refractive index data of all samples were determined using a variable angle spectroscopic ellipsometer (VASE, J.A. Woollam Co.) in a vertical configuration, within an illumination spot size of a few square mm. Ellipsometry quantities (ψ, Δ, and depolarization) were collected at three different angles of incidence (range of 60–75°) in the wavelength range of 300–1000 nm, and experimental data were fitted with WVASE32 software using a Cauchy model. The mean-squared error (MSE) is the quality estimator of the fitting procedure and it is calculated as a sum of squares of differences between modeled and measured data. A regression algorithm is applied for calculating and adjusting the variable parameters from model and experimental data to a minimum value of MSE.
As described by Iler [22], the assembly in thin films containing nanoparticles results in the presence of nanopores that lowers the effective refractive index of multilayers. When a difference in refractive index is observed for coating layers of varying thickness, it implies that either film porosity or relative amount of TiO2 to SiO2 nanoparticles, or both, differ. However, in our system, invariance of the refractive index with film thickness was observed and indicates that the composition of the layer for each system does not vary with final solution concentration, given a constant SiO2 to TiO2 ratio.
Ellipsometry can be used to estimate the porosity of thin films based on the assumption that the refractive index of the constituent materials is known [23,24,25]. When the constituent materials are nanoparticles, however, it is difficult to obtain reliable information on the refractive index of the nanoparticles utilized to create the film. The physical properties of nanoparticles differ from the bulk properties of their corresponding materials because of quantum confinement effects and large specific surface areas.
Rubner et al. have developed a method based on ellipsometry to determine the porosity of nanoporous thin films without any assumption about the material properties (i.e., refractive index) [26, 27]. For porous thin films, if the refractive index of the material’s framework and the overall porosity are unknown, then the solving of two independent equations containing the two parameters would be necessary to determine these values. These two independent equations can be obtained by measuring the values of the effective refractive index of the porous thin films in two different media of known refractive index (in our case, air and water), assuming that the thickness of the porous thin films remains constant in these two different media (thus, volume is constant). Another assumption is that pores are interconnected so that the chosen media can infiltrate and completely fill the pores. Based on the above arguments, the porosity and the refractive index of the film’s solid framework are expressed by:
where p represents the porosity (or fraction of void volume) of the thin films, and nf,air(=1.00), nf,water(=1.33), and nf,framework represents the refractive index of air, water, and the solid framework, respectively. nf,1 and nf,2 are the experimentally measured effective refractive indices of the porous thin films in media 1 (air) and 2 (water), respectively. The above equations have allowed for the determination of the thin film porosity and of the refractive index of the nanoparticle framework with ellipsometric measurements in the two different media, given the evidence that the solvents used during measurements effectively filled the pores, but did not swell the structure.
2.2.3 UV–Vis spectroscopy
The optical properties of the films deposited on silica glass slides were determined by recording their transmittance spectra in the 190−900 nm spectral range with a UV–Vis spectrophotometer (Jasco V-570), with a resolution of 0.1 nm.
2.2.4 Atomic force microscopy (AFM)
Morphological characterization of the sample surfaces was carried out by AFM (NT-MDT, Russia) in contact mode. Topology recordings were collected to provide estimates of the surface roughness of the produced films in sample areas of a few square μm dimension.
2.2.5 WCA measurement and analysis
The surface hydrophilicity of the coating was quantified from measurements of the WCA. The WCA was studied as a function of the key parameters: the concentration C of TEOS, the porosity, the concentration of SiO2 and TiO2 nanoparticles, and the aging of the solution. The contact angles presented were measured with a homemade apparatus [28], composed of a high-resolution camera, equipped with a telecentric objective. The sample was positioned on a sample stage, its vertical and horizontal positions finely adjusted with manual translation stages. A droplet of 500 nL was gently placed on the surface by a syringe, mounted on a vertical syringe pump. The drop was illuminated by a blue LED backlight, providing good contrast to image the droplet shape. Collected images were analyzed offline through a LabVIEW program. The program computes the droplet profile comparing the pixel greyscales of the image with selected thresholds. The profile was fitted by two polynomial functions of tuneable degree (one for the left, one for the right side of the droplet) and the derivates in the points of intersection between the fitted curves and the surface plane are calculated. The two contact angles are evaluated from the slope of the derivatives. In order to characterize a surface, data were collected from at least five droplets in different positions on the surface. The contact angle was expressed as an average of the different values obtained from every droplet, and the standard deviation is representative of the surface uniformity.
2.2.6 Nanowicking
Both surface roughness and surface porosity can influence the wetting behavior of a film. However, Rubner et al. [6] have observed that a superhydrophilic behavior is only obtained upon the establishment of a critical volume capacity, for a 3D interconnected pore network of the film, which ultimately gives rise to superhydrophilicity. Thus, the primary mechanism driving superhydrophilicity is the rapid liquid infiltration (nanowicking) in a wettable 3D interconnected nanoporous network, while surface roughness plays a secondary role. According to Quéré and co-workers; [29,30,31], the critical contact angle, θc, below which penetration of the surface texture by a liquid takes place is given by:
where ΦS is the solid fraction remaining dry during a wicking process and r (≥1) is the surface roughness (ratio of the actual surface area over the projected surface area). For a porous surface, r goes to infinity, and this equation predicts that the microstructure will be fully invaded by any liquid having a contact angle (on the flat surface) of less than 90°.
3 Results and discussion
3.1 The drying process
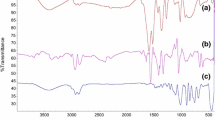
As-deposited films were thermally treated for 30 min at 80 °C to study the role of thermal activation of the layer. Drying process data were obtained by FT-IR spectroscopy measurements. A representative sample is chosen to show the evolution of the IR bands in the wavelength range of interest during the drying process. As reported in Fig. 1, the increase of the condensed TEOS signal at 1087 cm−1 and the reduction of the Si–OH signal at 968 cm−1 are clearly revealed. These data are consistent with a condensation mechanism promoted by the thermal treatment. The effect of increasing TEOS content with respect to silica nanoparticle concentration was monitored by FT-IR spectroscopy on a sample series thermally treated at 80 °C for 30 min. As shown in Fig. 2, the higher the TEOS concentration, the stronger the signal. A shift towards lower wavenumber was also observed in the case of the TEOS peak.
The hypothesis is sustained also by obtained porosity data reported in paragraph 3.1.2: the more condensed the film, the lower the pore concentration.
3.2 Porosity assessment
The porosity assessment was performed through software analysis of ellipsometry data based on the assumption that the framework formed by condensed TEOS and silica nanoparticles has a unique refractive index. While the determination of absolute porosity might be overestimated (the refractive index of the framework might be lower than the refractive index of fully condensed silica), the porosity fraction estimates can be used to compare samples with the similar framework (TEOS + LS nanoparticles) in order to correlate this information with WCA measurements (Fig. 3). The comparison is now limited to “all-silica” samples (the introduction of TiO2 in the framework requires a two-media determination). The assembly of nanoparticles in films results in the presence of nanopores. Silica nanoparticles can be thought to be dispersed in a silica matrix formed during TEOS condensation: the higher the amount of TEOS available, the thicker and the less porous the matrix. Nanopore capillary filling drives the reduction of WCA from 70° to a complete wetting condition.
3.3 Optical properties
The transmittance spectra for samples with silica and with a mixture of silica and titania nanoparticles are shown in Fig. 4. For all samples except the one containing TiO2 nanoparticles (40 mol%), the transmission is higher with respect to the bare glass substrate spectrum. A scattering contribution for short wavelengths (λ < 450 nm) is appreciable especially for the sample containing TiO2 nanoparticles. This is consistent with the higher refractive index of TiO2 as compared to SiO2, even though the particles are of comparable size. The same behavior has been observed irrespectively of the chosen SiO2 nanoparticles (LS or TMA). Transmittance is increased by 2% when coating only one side of the glass slide with a SiO2 layer so that a 4% increase is expected when the substrate is coated on both surfaces. By proper choice of layer thickness, antireflective properties can be obtained.
UV–Vis transmittance spectra at normal incidence for a set of prepared films on glass. The bare glass transmittance spectrum is reported for comparison. Reported spectra are relative to films characterized by a [TEOS]:[LS nanoparticles] molar ratio of 0.1:1, 0:5: 1, 1:1 and 1:1 in the presence of TiO2 nanoparticles (40mol%)
3.4 Surface roughness
AFM images of four representative samples were collected to provide information on the surface roughness of the deposited films: the higher the RNP, defined as the ratio [TEOS]/[nanoparticle], the lower the surface roughness (Table 1). On the one hand, the greater content of TEOS forms a compact matrix during condensation, in which LS nanoparticles are embedded. Their surface area is thus lowered by high amounts of TEOS, reducing the enhancement of superhydrophilic properties due to roughness. On the other hand, the addition of a small amount (40 mol%) of TiO2 nanoparticles completely changes the surface of the coating, thus compensating for the flattening effect of high TEOS concentration. While high TEOS content might be useful for conferring mechanical resistance to the coating, it would in turn reduce anti-fog properties. TiO2 nanoparticle addition represents a valuable alternative. Nonetheless, mechanical resistance might be poorer due to the lack of Ti–O–Si chemical bonds, which are promoted only at temperatures higher than 350 °C.
3.5 Layer thickness and optical constants
We report in Fig. 5 an estimate of the refractive index at the fixed wavelength (λ = 600 nm) for a coating comprising only silica nanoparticles (LS) and condensed TEOS binder. A low refractive index is obtained for small binder concentrations. Thickness increases fairly linearly with the TEOS concentration, as a result of an augmented amount of binder.
3.6 WCA: role of TEOS and TiO2 nanoparticle concentration
The surface hydrophilicity of the coating was quantified from measurements of the WCA.
The WCA was studied as a function of the concentration of TEOS. WCA increases with increasing TEOS concentration in the MS (Fig. 6). This is explained by the higher condensation degree reached by the coating with higher RNP; the silica nanoparticles (LS) can be thought to be dispersed in a silica matrix formed during TEOS condensation; the higher the amount of TEOS available, the thicker and the less porous the matrix. This is also shown by the correlation of porosity and WCA as presented earlier (paragraph 3.1.1).
We have also demonstrated for the sample with the highest RNP (RNP = 1, black square), that the WCA can be significantly reduced by introducing, after hydrolysis, a small amount of TiO2 nanoparticles to the MS (1.0, red circle). Both the different porosity and the modified surface chemistry introduced by the presence of TiO2 nanoparticles might play an important role. Thus, the evolution of WCA (Fig. 7) was studied for a sample characterized by a [TEOS]:[LS nanoparticles] molar ratio of 0.5:1 adding different amounts of TiO2 nanoparticles. The same samples were investigated for hydrophilicity restoring as explained above. A small amount of TiO2 changes the WCA properties irrespectively from the starting MS. It was found that a “minimum necessary” amount of TiO2 was required to give a superhydrophilic behavior and that increasing the UV-active nanoparticle concentration reduced the time for anti-fog restoring. In contrast, there is a linear increase of refractive index due to TiO2 presence which might raise light absorption and decrease antireflectivity. The correct amount of TiO2 must therefore be chosen to bear in mind restoring time, transparency, and antireflection properties.
3.7 WCA as a function of aging time
Samples were aged under atmosphere conditions for 7 weeks and WCAs were periodically measured to study the effects of natural aging on the film wettability. Three of the most representative sets are reported in Fig. 8, showing the evolution of the WCA. Most notable is the retention of the superhydrophilic properties for 30 days and beyond for the samples containing only silica. As expected, the samples containing TiO2 suffer from early contamination of the coating with a steep increase in the WCA already in the first few weeks. This drawback is compensated by the photodecomposition properties of TiO2, described above. A restoration method for all-silica samples is also proposed.
3.8 Study of surface nanostructure of another set of silica nanoparticles
Other samples were synthesized starting from a different SiO2 nanoparticle solution (TMA nanoparticles) and studied in order to understand the role of nanoparticle dimension in the superhydrophilic coating development. Results are presented for the equivalent concentration of active TiO2 solution (Fig. 9). The addition of titania does not reduce the initial WCA as in the LS sample (compare to Fig. 7) but instead, it brings the WCA to a plateau around 15° as well as a constant refractive index of 1.58 for high TiO2 content. This behavior can be justified by considering the packing geometry of two hard spheres with different radius: the smaller 14 nm TiO2 nanoparticles “fill” the pores left by the bigger 22 nm SiO2 until a constant volumetric ratio is reached (%mol TiO2 > 0.6); this, in turn, increases the refractive index (an indicator of porosity) and WCA to a constant value. The behavior is somewhat similar when silica nanoparticles of the same size as titania nanoparticles (12 nm of diameter) are added (Fig. 10): by mixing two different kinds of silica nanoparticles we can control WCA, thickness, and refractive index. The achieved tuneability is important for a proper coupling with other coating functionalities, such as, for example, antireflectivity.
3.9 Superhydrophilicity restoring
3.9.1 Estimation of pollutant concentration on surfaces
Pollutant concentration on the samples was monitored after 7 weeks of exposure to the laboratory environment (Fig. 11, left). The integrated C–H stretching area in the FT-IR spectrum (3000–2700 cm−1) was registered and correlated to the measured WCA. Evident trends are shown: the higher the TiO2 content, the higher the contact angle and the more augmented the surface contamination. This measurement confirms higher surface energy for TiO2, which can be readily polluted by environmental carbon.
3.9.2 Simulation with stearic acid and olive oil
Pollutant concentration was simulated with either stearic acid or olive oil, in order to yield a reproducible test for sample contamination, with substances having a similar composition to fingerprints, and sample restoration (Fig. 11, right). Concentrations as much as 10 times higher of contaminant are deposited through spin coating or simple cloth coating in order to simulate everyday use of the substrates. Collected data are reported in the right plot of Fig. 11: the contact angle still follows the same trend, i.e., the higher the C–Hn stretching area, the higher the WCA.
3.9.3 Pollutant decomposition
In order to demonstrate the functionality of the coating with TiO2 nanoparticles, sets of samples were produced varying the amount of added TiO2. The goal is to find the necessary amount of active components to give the desired self-cleaning properties. The samples were exposed to UV light (Mercury Quartz Lamp, λmax = 365 nm, 0.9 mW cm−2) for different time intervals to follow the degradation behavior. Again, the C–H stretching area was measured to monitor the evolution of the samples (Fig.12). It can be noticed that the higher the TiO2 content, the faster the pollutant degradation. The analyzed samples completed the degradation process within 40 min of UV exposure.
In order to improve the performance of the proposed coating, TMA SiO2 nanoparticles of 22 nm diameter were used for a representative sample, prepared to start from the previous findings. Namely, TEOS and TiO2 concentration were weighted by nanoparticle surface to have comparable results. In Fig. 13, FT-IR spectra for samples comprising LS and TMA nanoparticles show the progressive decomposition of stearic acid. The decomposition of stearic acid proceeds faster in the sample with TMA nanoparticles (right plot), for a comparable surface coverage of TiO2 nanoparticles. Porosity might play a decisive role. The shape and intensity of the –OH composite band is evolving during UV exposure. The signal at 3400 cm−1 might be due to Ti–OH, the creation of which is promoted by UV (photo-induced superhydrophilicity) in the presence of H2O. As shown in Fig. 14, the decomposition of stearic acid in the sample with TMA proceeds faster (compare with spectra of Fig. 12).
3.9.4 Anti-fog restoring
Another method we considered for superhydrophilicity and anti-fog restoring was sample immersion in a 5% water solution of NaClO. The samples prepared with LS nanoparticles, [TEOS]/[SiO2] molar ratio of 0.5:1, were previously contaminated with olive oil spread on the coating surface with the help of paper cloth, and then they were immersed for 10 min in the solution. WCA measurements, before and after the restoring step, confirm the possibility of cleaning the coated glass even without active titania particles in the coating composition. The two methods (UV restoring and wet restoring) are compared in Fig. 15. The connection between the two restoring methods is the cleaning of the surface via a mild oxidizing agent (TiO2 under UV in the presence of H2O, and NaClO in H2O). Further strategies can be drawn starting from this simple mechanism.
4 Conclusions
Highly porous nanocomposite films were obtained at room temperature by choosing the mean diameter of the constituent nanoparticles, controlling and optimizing the proper amount of binder (TEOS), mixing families of nanoparticles with different diameters (12–22 nm), and adding TiO2 nanoparticles. This methodology allowed for a wide range of available refractive indices during mixing with the titania phase; this tunability is important for proper coupling with antireflectivity or with other layers to produce multifunctional coatings.
Superhydrophilic properties were retained by the samples over 30 days, a relatively long time if compared with literature results. In order to experimentally mimic surface pollution, the surface of the sample was coated with olive oil using wet clothing and with stearic acid by spin coating. A strong correlation was found between the integrated FT-IR signal over the C–Hn stretching area and the measured WCA, both for stearic acid and manual oil staining.
For the restoration of the superhydrophilic behavior, two strategies were investigated. First, the presence of TiO2 nanoparticles permitted, under UV illumination, a decomposition with the time of organic pollutants deposited over the surface. Moreover, photo-induced hydrophilicity enhanced anti-fog properties after UV exposure. A second strategy involved the immersion of the samples in a 5% NaClO water solution, to effectively clean the surface in 10 min. Optimization of the NaClO amount and immersion times can be done to meet “safe handling” criteria.
References
Howarter JA, Yougblood JP (2008) Macromol Rapid Commun 29:455–466
Wenzel RN (1936) Ind Eng Chem 28:988
Wenzel RNJ (1949) Phys Colloid Chem 53:1466
Cassie ABD, Baxter S (1944) Trans Faraday Soc 40:546
José B, Uwe T, David Q (2002) Colloids Surf A: Physicochem Eng Asp 206:41–46
Cebeci FC, Wu Z, Zhai L, Cohen RE, Rubner MF (2006) Nanoporosity-driven superhydrophilicity: a means to create multifunctional antifogging coatings. Langmuir 22:2856–2862
Takeuchi M, Sakamoto K, Martra G, Coluccia S, Anpo M (2005) Mechanism of photoinduced superhydrophilicity on the TiO2 photocatalyst surface. J Phys Chem B 109(32):15422–15428
Permpoon S, Berthomé G, Baroux B, Joud JC, Langlet M (2006) Natural superhydrophilicity of sol–gel derived SiO2–TiO2 composite films. J Mater Sci 41(22):7650–7662
Guan K, Lu B, Yin Y (2003) Enhanced effect and mechanism of SiO2 addition in super-hydrophilic property of TiO2 films. Surf Coat Technol 173(2-3):219–223
Wang S, Liu K, Yao X, Jiang L (2015) Bioinspired surfaces with superwettability: new insight on theory, design, and applications. Chem Rev 115(16):8230–8293
Hinze P, Dislich H (1986) Antireflecting light-scattering coatings via the sol–gel-procedure. J Non-Cryst Solids 82:411–416
Yoldas BE, Partlow DP (1984) Wide spectrum antireflective coating for fused silica and other glasses. Appl Opt 23:1418–1424
Uhlmann DR, Suratwala T, Davidson K, Boulton JM, Teowee G (1997) Sol–gel derived coatings on glass. J Non-Cryst Solids 218:113–122
Dal Zilio S, Della Giustina G, Brusatin G, Tormen M (2010) Microlenses arrays on large area UV transparent hybrid sol–gel materials for optical tools. Microelectron Eng 87:1143–1146
Yu J, Zhou M, Yu H, Zhang Q, Yu Y (2006) Enhanced photoinduced super-hydrophilicity of the sol–gel-derived TiO2 thin films by Fe-doping. Mater Chem Phys 95(2–3):193–196
Eshaghi A, Dashti A, Eshaghi A, Mozaffarinia RM(2011) Photo-induced superhydrophilicity of nanocomposite TiO2–SiO2 thin film Sci Pol 29:22
Galeener FL (1979) Phys Rev B 19:4292
Galeener FL, Lucovsky G (1976) Phys Rev Lett 37:1474
Berreman DW (1963) Phys Rev 130:2193
Plinio I (2003) Infrared spectroscopy of sol–gel derived silica-based films: a spectra-microstructure overview. J Non-Crystalline Solids 316:309–319
Antonello A, Brusatin G, Guglielmi M, Bello V, Mattei G, Zacco G, Martucci A (2011) Nanocomposites of titania and hybrid matrix with high refractive index. J Nanopart Res 13:1697–1708
Iler RK (1966) J Colloid Interface Sci 21:569–594
H Fujiwara (2007) Spectroscopic ellipsometry: principles and applications. John Wiley & Sons, Ltd.
Brigo L, Michieli N, Artiglia L, Scian C, Rizzi GA, Granozzi G, Mattei G, Martucci A, Brusatin G (2014) Silver nanoprism arrays coupled to functional hybrid films for localized surface plasmon resonance-based detection of aromatic hydrocarbons. ACS Appl Mater Interfaces 6(10):7773–7781
A. Antonello, B. Jia, Z. He, D. Buso, G. Perotto, L. Brigo, G. Brusatin, M. Guglielmi, M. Gu, A. Martucci optimized electroless silver coating for optical and plasmonic applications plasmonics. 12/2012
Lee D, Rubner MF, Cohen RE (2006) All-nanoparticle thin-film coatings. Nano Lett 6(10):2305–2312
Brigo L, Gazzola E, Cittadini M, Zilio P, Zacco G, Romanato F, Martucci A, Guglielmi M, Brusatin G (2013) Short and long range surface plasmon polariton waveguides for xylene sensing. Nanotechnology 24:155502
Tóth T, Ferraro D, Chiarello E, Pierno M, Mistura G, Bissacco G, Semprebon C (2011) Suspension of water droplets on individual pillars. Langmuir 27(8):4742–4748
Bico J, Marzolin C, Quere D (1999) Europhys Lett 47:743.
Quere D (2001) Europhys Lett 55:214
Bico J, Thiele U, Quere D (2002) Colloids Surf A 206:41
Acknowledgements
We thank Dr. Federico Menta and Dr. Hickenboth, Charles R.—PPG Industries—for the industrial support through the project “Hydrophilic/antifog surfaces based on nanostructured oxide” and for the scientific discussion. Prof. Giampaolo Mistura and Dr. Silvia Varagnolo are gratefully acknowledged for their assistance in performing water contact angle measurements and data analysis.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Momoli, R., Giacomazzo, S., Gandin, A. et al. Anti-fog nanocomposite coatings of enhanced durability. J Sol-Gel Sci Technol 101, 46–57 (2022). https://doi.org/10.1007/s10971-021-05554-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05554-6