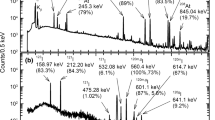
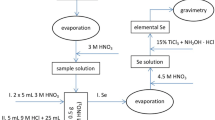
Abstract
Radio-chromatography was conducted by using the astatine radionuclides 209,210,211At produced in the 7Li induced reaction of 209Bi. The speciation of dissolved astatine chemical species of astatide (At−), astatate (AtO3−) and perastatate (AtO4−) was carried out by thin layer chromatography on silica gel with an ethanol/water solution. Amounts of the astatine species varied with concentrations of oxidizing and reducing reagents, potassium periodate, sodium sulfate and hydrazine hydrate. The oxidation–reduction reactions between At−(−I) and AtO3− (V) were found to form volatile At0 (0) and release it from a silica gel thin layer in the development.







Similar content being viewed by others
References
Wilbur DS (2013) Enigmatic astatine. Nat Chem 5:246
Johnson GL, Leininger RF, Segre E (1949) Chemical properties of astatine. I. J Chem Phys 17:1–10
Appelman EH (1960) The radiochemistry of astatine. Natl Acad Sci. Natlional Research Council, Nuclear Science Series, U S Atomic Energy Commission
Rössler K, Tornau W, Stöcklin G (1974) Rapid separation of carrier-free inorganic and organic compounds of radioiodine and astatine by high-pressure liquid chromatograpy. J Radioanal Nucl Chem 21:199–209
Dreyer I, Dreyer R, Norseev YV, Chalkin VA (1978) Synthese anorganischer astatverbindungen und untersuchung ihrer eigenschaften mittels papierelektrophorese und paierchromatographie. Radiochem Radioanal Lett 33:291–300 [in German]
Visser GWM, Diemer EL (1983) Inorganic astatine chemistry: formation of complexes of astatine. Radiochim Acta 33:145–151
Milanov M, Deberenz V, Khalkin VA, Marinov A (1984) Chemical properties of positive singly charged astatine ion in aqueous solution. J Radioanal Nucl Chem 83:291–299
Dreyer R, Dreyer I, Fischer S, Hartmann H, Rösch F (1985) Synthesis and characterization of cationic astatine compounds with sulpher-containing ligands stable in aqueous solutions. J Radioanal Nucl Chem 96:333–342
Ludwig R, Fischer S, Hussein H, Frind M, Dreyer R (1989) Stability constants of At(I)-complexes with thiourea, iodide and mixed ligands in ethanol and water. J Radioanal Nucl Chem 134:141–149
Champion J, Alliot C, Huclier S, Deniaud D, Asfari Z, Montavon G (2009) Determination of stability constants between complexing agents and At(I) and At(III) species present at ultra-trace concentrations. Inorg Chim Acta 362:2654–2661
Champion J, Alliot C, Renault E, Mokili BM, Chérel M, Galland N, Montavon G (2010) Astatine standard redox potentials and speciation in acidic medium. J Phys Chem A 114:576–582
Campion J, Sabatié-Gogova A, Bassal F, Ayed T, Alliot C, Gallland N, Montavon G (2013) Investigation of astatine(III) hydrolyzed species: experiments and relativistic calculations. J Phys Chem A 117:1983–1990
Balkin EB, Hamlin DK, Gagnon K, Chyan MK, Pal S, Watanabe S, Wilber DS (2013) Evaluation of a wet chemistry method for isolation of cyclotron produced [211At]astatine. Appl Sci 3:636–655
Nishinaka I, Hashimoto K, Suzuki H (2018) Thin layer chromatography for astatine and iodine in solutions prepared by dry distillation. J Radioanal Nucl Chem 318:897–905
Nishinaka I, Yokoyama A, Washiyama K, Maeda E, Watanabe S, Hashimoto K, Ishioka NS, Makii H, Toyoshima A, Yamada N, Amano R (2015) Production and separation of astatine isotopes in the 7Li + natPb reaction. J Radioanal Nucl Chem 304:1077–1083
Yordanov AT, Pozzi O, Carlin S, Akabani G, Wieland B, Zalutsky MR (2004) Wet harvesting of no-carrier-added 211At from an irradiated 209Bi target for radiopharmaceutical applications. J Radioanal Nucl Chem 262:593–599
Acknowledgements
The authors thank the crew of the JAEA Tandem Accelerator for accelerator operation. We are thankful to Dr. M. Asai for his help for γ-ray and α-ray spectroscopies and H. Saeki for visualizing imaging plates by BAS. This work was supported by JSPS KAKENHI Grant Numbers JP2360013, JP15K04741 and JP18K11939.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nishinaka, I., Hashimoto, K. & Suzuki, H. Speciation of astatine reacted with oxidizing and reducing reagents by thin layer chromatography: formation of volatile astatine. J Radioanal Nucl Chem 322, 2003–2009 (2019). https://doi.org/10.1007/s10967-019-06900-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06900-3