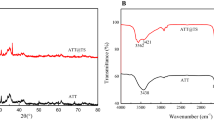
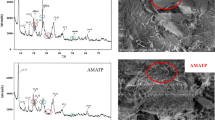
Abstract
Effective adsorption of Sr(II) onto H2O2-modified attapulgite in aqueous solution was investigated about kinetics and isothermal equilibrium adsorption. The adsorption equilibrium process of Sr(II) on adsorbents reached about 8 h at 40 °C. The adsorption kinetics followed the pseudo-second order equation and the isothermal adsorption data were fit well with the Langmuir isotherm model. The enhanced adsorption mechanism of H2O2-modified attapulgite for Sr(II) in aqueous solution was expatiated in detail. The H2O2 treatment for attapulgite is effective and as-made adsorbents can be applied for removal of Sr(II) in radioactive waste water.









Similar content being viewed by others
References
Amphlett CB, McDonald LA (1958) Equilibrium studies on natural ion-exchange minerals-III: caesium, sodium and ammonium ions. J Inorg Nucl Chem 6:145–152
Brook BW, Bradshaw CJA (2015) Key role for nuclear energy in global biodiversity conservation. Conserv Biol 29:702–712
Napier BA (2014) Joint U.S./Russian studies of population exposures resulting from nuclear production activities in the Southern Urals. Health Phys 106:294–304
Scourse E, Aspinall WP, Chapman N (2014) Using expert elicitation to characterise long-term tectonic risks to radioactive waste repositories in Japan. J Risk Res 18:364–377
Martín LB, Wolters R, Rutqvist J, Lux KH, Birkholzer JT (2015) Comparison of two simulators to investigate thermal–hydraulic–mechanical processes related to nuclear waste isolation in saliferous formations. Comput Geotech 66:219–229
O’Hara MJ, Burge SR, Grate JW (2009) Automated radioanalytical system for the determination of 90Sr in environmental water samples by 90Y cherenkov radiation counting. Anal Chem 81:1228–1237
Snow MS, Snyder DC, Clark SB, Kelley M, Delmore JE (2015) 137Cs activities and 135Cs/137Cs isotopic ratios from soils at idaho national laboratory: a case study for contaminant source attribution in the vicinity of nuclear facilities. Environ Sci Technol 49:2741–2748
Grahek Ž, Košutić K, Lulić S (1999) Improved method for the separation of radioactive strontium from various samples by mixed solvent anion exchange. J Radioanal Nucl Chem 242:33–40
Sansone F, Fontanella M, Casnati A, Ungaro R, Böhmer V, Saadioui M, Liger K, Dozol JF (2006) CMPO-substituted calix[6]- and calix[8]arene extractants for the separation of An3+/Ln3+ from radioactive waste. Tetrahedron 62:6749–6753
Boudriche L, Calvet R, Hamdi B, Balard H (2011) Effect of acid treatment on surface properties evolution of attapulgite clay: an application of inverse gas chromatography. Colloids Surf A 392:45–54
Campelo JM, Garcia A, Luna D, Marinas JM (1990) Cyclohexene skeletal is omerization activity of sepiolites modified with B3+ or Al3+ ions. React Kinet Catal Lett 41:13–19
Omri A, Mabrouk NB, Sassi-Tmar A (2015) Modeling the causal linkages between nuclear energy, renewable energy and economic growth in developed and developing countries. Renew Sust Energy Rev 42:1012–1022
Pan J, Zou X, Yan Y, Wang X, Guan W, Han J, Wu X (2010) An ion-imprinted polymer based on palygorskite as a sacrificial support for selective removal of strontium (II). Appl Clay Sci 50:260–265
Wang W, Gu Z, Gao X, Jiang H, Liu W (2014) From natural attapulgite to phosphor materials: characterization, photoluminescence and structure. Mater Res Bull 56:8–11
Kim K, Kim K, Choi M, Son SH, Han JH (2012) Treatment of ion exchange resins used in nuclear power plants by super- and sub-critical water oxidation- A road to commercial plant from bench-scale facility. Chem Eng J 189–190:213–221
Ambashta Ritu D, Sillanpää MET (2012) ‘Membrane purification in radioactive waste management: a short review. J Environ Radioact 105:76–84
Zakrzewska-Trznadel G, Harasimowicz M, Chmielewski AG (2001) Membrane processes in nuclear technology-application for liquid radioactive waste treatment. Sep Purif Technol 22–23:617–625
Matsuo T, Nishi T (2000) Activated carbon filter treatment of laundry waste water in nuclear power plants and filter recovery by heating in vacuum. Carbon 38:709–714
Valsalaa TP, Roy SC, Shah JG, Gabriel J, Raj K, Venugopal V (2009) Removal of radioactive caesium from low level radioactive waste (LLW) streams using cobalt ferrocyanide impregnated organic anion exchanger. J Hazard Mater 166:1148–1153
Katz A, Brough AR, Kirkpatrick RJ, Struble LJ, Sun GK, Young JF (2001) Cement solidification of simulated off-gas condensates from vitrification of low-level nuclear waste solutions. Waste Manag 21:543–553
Li J, Wang J (2006) Advances in cement solidification technology for waste radioactive ion exchange resins: a review. J Hazard Mater B135:443–448
Loiseau P, Caurant D (2010) Glass–ceramic nuclear waste forms obtained by crystallization of SiO2–Al2O3–CaO–ZrO2–TiO2 glasses containing lanthanides (Ce, Nd, Eu, Gd, Yb) and actinides (Th): study of the crystallization from the surface. J Nucl Mater 402:38–54
Bradley WF (1940) The structural scheme of attapulgite. Am Mineral 25:405–410
Zhang ZZ, Sparks DL, Scrivner NC (1993) Sorption and desorption of quaternary amine cations on clays. Environ Sci Technol 27:1625–1631
Serna C, Vanscoyoc GE, Ahlrichs JL (1977) Hydroxyl groups and water in palygorskite. Am Mineral 62:784–792
Tang Q, Wang F, Guo H, Yang Y, Du Y, Liang J, Zhang F (2015) Effect of coupling agent on surface free energy of organic modified attapulgite (OAT) powders and tensile strength of OAT/ethylene-propylene-diene monomer rubber nanocomposites. Powder Technol A 270:92–97
Li X, Yan C, Luo W, Gao Q, Zhou Q, Liu C, Zhou S (2016) Exceptional cerium(III) adsorption performance of poly(acrylic acid) brushes-decorated attapulgite with abundant and highly accessible binding sites. Chem Eng J 284:333–342
Xiao F, Gu YJ, Tang Z, Han F, Shao JL, Xu Q, Zhu HL (2015) ZrO2 modified MnOx/attapulgite catalysts for NH3-SCR of NO at low temperature. J Chem Eng Jpn 48:481–487
Xavier KCM, Santos MDSFD, Santos MRMC, Oliveira MER, Carvalho MWNC, Osajima JA, Filho ECDS (2014) Effects of acid treatment on the clay palygorskite: XRD, surface area, morphological and chemical composition. Mater Res 17:3–18
Liu Q, Cheng M, Long Y, Yu M, Wang T, Jiang G (2014) Graphenized pencil lead fiber: facile preparation and application in solid-phase microextraction. J Chromatogr A 1325:1–7
Mamina AK, Kotelnikova EN, Muromtsev VA, Frankkamenetskii V (1990) Influence of the structural perfection of phlogopite crystals on their cleavability by hydrogen peroxide. Inorg Mater 26:2104–2107
Liu DC, Xiong XL, Yang WB (2010) Research on synthesis and adsorption performance test of nano-ZnO/attapulgite clay composite materials. Non-Met Mines 33:74–77
Deng Y, Wu F, Liu B, Hu X, Sun C (2011) Sorptive removal of β-blocker propranolol from aqueous solution by modified attapulgite: effect factors and sorption mechanisms. Chem Eng J 174:571–578
Lin J, Zhan Y (2012) Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem Eng J 200–202:202–213
Yi R, Ye G, Wu FC, Lv DC, Chen J (2016) Magnetic solid-phase extraction of strontium using core–shell structured magnetic microspheres impregnated with crown ether receptors: a response surface optimization. J Radioanal Nucl Chem 308:599–608
Takahashi Y, Minai Y, Ambe S, Makide Y, Ambe F (1999) Comparison of adsorption behavior of multiple inorganic ions on kaolinite and silica in the presence of humic acid using the multitracer technique. Geochim Cosmochim Acta 63:815–836
Yılmaz MS, Özdemir ÖD, Pişkin S (2015) Synthesis and characterization of MCM-41 with different methods and adsorption of Sr2+on MCM-41. Res Chem Intermed 41:199–211
Chu Z, Liu J, Han C (2015) Removal of strontium ions from aqueous solution using hybrid membranes: kinetics and thermodynamics. Chin J Chem Eng. 23:1620–1626
Villard A, Siboulet B, Toquer G, Merceille A, Grandjean A, Dufrêche JF (2015) Strontium selectivity in sodiumnonatitanate Na4Ti9O20·xH2O. J Hazard Mater 283:432–438
Moller T, Clearfield A, Harjula R (2002) Preparation of hydrous mixed metal oxides of Sb, Nb, Si, Ti and W with a pyrochlore structure and exchange of radioactive cesium and strontium ions into the materials. Microporous Mesoporous Mater 54:187–199
Zhang L, Wei J, Zhao X, Li F, Jiang F (2015) Adsorption characteristics of strontium on synthesized antimony silicate. Chem Eng J 277:378–387
Zhang L, Wei J, Zhao X, Li F, Jiang F, Zhang M (2015) Strontium(II) adsorption on Sb(III)/Sb2O5. Chem Eng J 267:245–252
Keçeli G (2015) Adsorption kinetics and equilibria of strontium onto kaolinite. Sep Sci Technol 50:72–80
Missana T, Garcia-Gutierrez M, Alonso U (2008) Sorption of strontium onto illite/smectite mixed clays. Phys Chem Earth 33:s156–s162
Galamboš M, Kufčáková J, Rajec P (2009) Sorption of strontium on Slovak bentonites. J Radioanal Nucl Chem 281:347–357
Chaalal O, Zekri AY, Soliman AM (2015) A novel technique for the removal of strontium from water using thermophilic bacteria in a membrane reactor. J Ind Eng Chem 21:822–827
Tiwari D, Lee SM (2015) Physico-chemical studies in the removal of Sr (II) from aqueous solutions using activated sericite. J Environ Radioact 147:76–84
Tu YJ, You CF, Chen YR, Huang CP, Huang YH (2015) Application of recycled iron oxide for adsorptive removal of strontium. J Taiwan Inst Chem E 53:92–97
Üçgül E, Girgin İ (2002) Chemical exfoliation characteristics of karakoc phlogopite in hydrogen peroxide solution. Turk J Chem 26:431–439
Huang J, Liu Y, Jin Q, Wang X, Yang J (2007) Adsorption studies of a water soluble dye, Reactive Red MF-3B, using sonication-surfactant-modified attapulgite clay. J Hazard Mater 143:541–548
Zou X, Pan J, Ou H, Wang X, Guan W, Li C, Yan Y, Duan Y (2011) Adsorptive removal of Cr(III) and Fe(III) from aqueous solution by chitosan/attapulgite composites: equilibrium, thermodynamics and kinetics. Chem Eng J 167:112–121
Corma A, Mifsud A, Sanz E (1987) Influence of the chemical composition and textural characteristics of palygorskite on the acid leaching of octahedral cations. Clay Miner 22:225–232
Jiang J, Duanmu C, Yang Y, Gu X, Chen J (2014) Synthesis and characterization of high siliceous ZSM-5 zeolite from acid-treated palygorskite. Powder Technol 251:9–14
Acknowledgements
This work was financially supported by the PhD Fund of South West University of Science and Technology (Granted No. 13zx7132).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, D., Zheng, H. Enhanced adsorption of radioactive strontium ions from aqueous solution by H2O2-modified attapulgite. J Radioanal Nucl Chem 311, 1883–1890 (2017). https://doi.org/10.1007/s10967-017-5184-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5184-3