Abstract
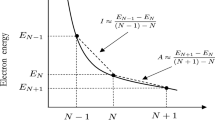
Chemical Reactivity Theory (CRT) contains reactivity indices defined as first and second derivatives of ground-state properties with respect to electron number such as the electronegativity and the hardness. This necessitates use of the Perdew, Parr, Levy, and Balduz (PPLB) version of noninteger density-functional theory (NIDFT) to provide a basis for CRT in DFT. However, the PPLB NIDFT yields ground-state properties which are piecewise linear continuous functions of number, yielding vanishing hardness and staircase electronegativities which do not admit electronegativity equalization. To overcome these difficulties, in the present paper we modify the relationship between CRT and DFT, basing the former on our previously formulated “atoms” in “molecules” theory (AIMT) but retaining the PPLB NIDFT. We recapture electronegativity equalization through the agency of a uniquely defined reactivity potential. We demonstrate that a positive definite hardness matrix can be defined which controls the minimum cost to the AIMT energy functional of internal fluctuations of the electron numbers of the parts of a system.
Similar content being viewed by others
Abbreviations
- AIMT:
-
“Atoms” in “Molecules” theory
- CP:
-
Car-Parinello(28)
- CRT:
-
Chemical-reactivity theory
- DF:
-
Density functional
- DFT:
-
Density-functional theory
- EDF:
-
Ensemble density functional
- EDFT:
-
Ensemble density-functional theory
- EEP:
-
Electronegativity equalization principle
- EVR:
-
Ensemble v-representable
- HK:
-
Hohenberg-Kohn(1)
- HOMO:
-
Highest occupied molecular orbital
- KS:
-
Kohn-Sham(2)
- LL:
-
Levy-Lieb constrained search algorithm(11–13)
- LUMO:
-
Lowest unoccupied molecular orbital
- NIDF:
-
Non-integer density functional
- NIDFT:
-
Non-integer density-functional theory
- PPLB:
-
Perdew Parr Levy and Balduz(9)
References
P. Hohenberg and W. Kohn, Phys. Rev. 136B:864 (1964).
W. Kohn and L. J. Sham, Phys. Rev. 140:A1133 (1965)
R. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York (1989).
R. F. Nalewajski, J. Korchowiec, and A. Michalak, Topics Curr. Chem. 183:25 (1996).
P. Geerlings, F. De Proft, and W. Langenaeker, Chem. Rev. 103:1793 (2003).
R. T. Sanderson, Science 114:670 (1951).
J. F. Janak, Phys. Rev. B 18:7165 (1978).
N. D. Mermin, Phys. Rev. 137:A1441 (1965).
J. P. Perdew, R. G. Parr, M. Levy, and J. R. Balduz, Jr., Phys. Rev. Lett. 49:1691 (1982).
J. P. Perdew, in Density Functional Methods in Physics, ed. R. M. Dreizler and J. da Providencia (Plenum, New York, 1985) p. 265.
M. Levy, Proc. Nat. Acad. Sci. USA 76:6062 (1979).
E. H. Lieb, in Physics as Natural Philosophy, eds. A. Shimony and H. Feshbach (MIT Press, Cambridge, 1982) p. 111.
E. H. Lieb, in Density Functional Methods in Physics, ed. R. M. Dreizler, NATO ASI Series B123 (Plenum, New York, 1985) p. 31.
R. G. Parr and R. G. Pearson, J. Am. Chem. Soc. 105:7512 (1983).
R. G. Pearson, J. Chem. Educ. 64:561 (1987).
In earlier papers [18–20] we have expressed doubts about the validity of the PPLB EDF, which we hereby retract.
Y. Zhang and W. Yang, Theor. Chem. Acc. 103:346 (2000).
M. H. Cohen and A. Wasserman, Israel J. Chem. 43:219 (2003).
M. H. Cohen, Topics Curr. Chem. 183:143 (1996).
M. H. Cohen and A. Wasserman, in Proceedings of the International School of Physics “Enrico Fermi” Course CLV: The Physics of Complex Systems (New Advances and Perspectives), eds. F. Mallamace and H. E. Stanley (IOS Press, Amsterdam, 2004) pp. 253–295.
R. G. Parr, P. W. Ayers, and R. Nalewajski, J. Phys. Chem. A 109:3957 (2005).
R. F. Nalewajski and R. G. Parr, Proc. Natl. Acad. Sci. USA 97:8879 (2000).
F. L. Hirshfeld, Theor. Chim. Acta 44:129 (1977).
S. M. Valone, J. Chem. Phys. 73:1344, 4653 (1980).
M. H. Cohen, unpublished.
P. Blanchard and E. Brüning, Variational Methods in Mathematical Physics (Springer, Berlin, 1982).
R. M. Dreizler and E. K. U. Gross, Density Functional Theory (Springer, Berlin, 1990).
R. Car and M. Parinello, Phys. Rev. Lett. 55:2471 (1985).
R. G. Parr and W. Yang, J. Am. Chem. Soc. 106:4049 (1984).
M. E. Mura, P. J. Knowles, and C. A. Reynolds, J. Chem. Phys. 106:9659 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cohen, M.H., Wasserman, A. On Hardness and Electronegativity Equalization in Chemical Reactivity Theory. J Stat Phys 125, 1121–1139 (2006). https://doi.org/10.1007/s10955-006-9031-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10955-006-9031-0