Abstract
The use of static and dynamic light scattering (SLS and DLS, respectively) for chraracterization of solutions (0.005–0.20 mol·L− 1) of a series of carbohydrate-based low-molecular-mass compounds (glycosyl donors for chemical glycosylation) in anhydrous acetonitrile is described. The DLS data obtained suggest that, in the cases studied, the solute molecules form different supramolecular assemblies (supramers) with variable hydrodynamic radii. Analysis of concentration dependences of complementary SLS data for these solutions revealed that solvent quality may change dramatically with concentration and with the nature of solute. These results suggest that a combination of SLS and DLS is a valuable analytical tool for the supramer analysis of reaction solutions, which is useful for the rational selection of optimal concentrations for performing glycosylation reactions.




Similar content being viewed by others
Notes
The use of light scattering could allow an extension of the suprmer analysis to solutions of achiral solutes.
For the purpose of supramer analysis, it is not critical whether the Rh values represent the “true” dimensions of light scattering particles or just give us some response function that reflects the situation in the solution in a complex way.
Note that only positive values of monotonically decreasing correlation function g1(τ) = g2(τ) – 1 can be correctly recalculated to hydrodynamic radii Rh values of “particles” dispersed in solvent. For this reason, only relatively small lag times (τ < 0.01 ms), at which correlation function is monotonically decreasing and assumes only positive values (until it becomes equal to zero), were considered for calculation of Rh values. The larger lag times cannot be considered for correct calculation of Rh values since the correlation function was increasing from zero before becoming positive. Therefore, at these greater lag times the correlation function can be considered as sign-alternating function, which is indicative of refractive index homogeneity of the medium and which corresponds to a structurally uniform solution.
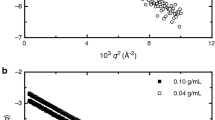
For solutions of compound 2 the negative slope for dilute solutions is also visible athough less clearly due to more considerable intensity scatter (see Fig. 4b).
Note that compound 2 has additionally a different anomeric configuration, which changes the orientation of hydrogen bond accepting methoxycarbonyl group from equatorial (in 1 and 3) to axial (in 2). For this reason, a direct comparative analysis of SLS/DLS data for solutions of compound 2 with data for solutions of compounds 1 and 3 is not possible.
References
Varki, A.: Biological roles of glycans. Glycobiology 27, 3–49 (2017). https://doi.org/10.1093/glycob/cww086
Fügedi, P.: Oligosaccharide synthesis. In: Levy, D.E., Fügedi, P. (eds.) The Organic Chemistry of Sugars, pp. 199–239. CRC Press, Taylor & Francis Group, Boca Raton (2005)
Demchenko, A.V. (ed.): Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance. Wiley-VCH, Weinheim (2008)
Carmona, A.T., Moreno-Vargas, A.J., Robina, I.: Glycosylation methods in oligosaccharide synthesis. Part 1. Curr. Org. Synth. 5, 33–60 (2008). https://doi.org/10.2174/157017908783497545
Carmona, A.T., Moreno-Vargas, A.J., Robina, I.: Glycosylation methods in oligosaccharide synthesis. Part 2. Curr. Org. Synth. 5, 81–116 (2008). https://doi.org/10.2174/157017908784221567
Smoot, J.T., Demchenko, A.V.: Oligosaccharide synthesis: from conventional methods to modern expeditious strategies. Adv. Carbohydr. Chem. Biochem. 62, 161–250 (2009). https://doi.org/10.1016/s0065-2318(09)00005-5
Crich, D.: Methodology development and physical organic chemistry: a powerful combination for the advancement of glycochemistry. J. Org. Chem. 76, 9193–9209 (2011). https://doi.org/10.1021/jo2017026
Yasomanee, J.P., Demchenko, A.V.: From stereocontrolled glycosylation to expeditious oligosaccharide synthesis. Trends Glycosci. Glycotechnol. 25, 13–42 (2013). https://doi.org/10.4052/tigg.25.13
Mydock, L.K., Demchenko, A.V.: Mechanism of chemical O-glycosylation: from early studies to recent discoveries. Org. Biomol. Chem. 8, 497–510 (2010). https://doi.org/10.1039/b916088d
Crich, D.: Mechanism of a chemical glycosylation reaction. Acc. Chem. Res. 43, 1144–1153 (2010). https://doi.org/10.1021/ar100035r
Bohé, L., Crich, D.: A propos of glycosyl cations and the mechanism of chemical glycosylation. Compt. Rend. Chim. 14, 3–16 (2011). https://doi.org/10.1016/j.crci.2010.03.016
Huang, M., Garrett, G.E., Birlirakis, N., Bohé, L., Pratt, D.A., Crich, D.: Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects. Nat. Chem. 4, 663–667 (2012). https://doi.org/10.1038/nchem.1404
Ranade, S.C., Demchenko, A.V.: Mechanism of chemical glycosylation: focus on the mode of activation and departure of anomeric leaving groups. J. Carbohydr. Chem. 32, 1–43 (2013). https://doi.org/10.1080/07328303.2012.749264
Bohé, L., Crich, D.: A propos of glycosyl cations and the mechanism of chemical glycosylation; the current state of the art. Carbohydr. Res. 403, 48–59 (2015). https://doi.org/10.1016/j.carres.2014.06.020
Adero, P.O., Amarasekara, H., Wen, P., Bohé, L., Crich, D.: The experimental evidence in support of glycosylation mechanisms at the SN1–SN2 interface. Chem. Rev. 118, 8242–8284 (2018). https://doi.org/10.1021/acs.chemrev.8b00083
van der Vorm, S., Hansen, T., van Hengst, J.M.A., Overkleeft, H.S., van der Marel, G.A., Codée, J.D.C.: Acceptor reactivity in glycosylation reactions. Chem. Soc. Rev. 48, 4688–4706 (2019). https://doi.org/10.1039/C8CS00369F
Kononov, L.O., Tsvetkov, D.E., Orlova, A.V.: Conceivably the first example of a phase transition in aqueous solutions of oligosaccharide glycosides. Evidence from variable-temperature 1H NMR and optical rotation measurements for a solution of allyl lactoside. Russ. Chem. Bull. 51, 1337–1338 (2002). https://doi.org/10.1023/a:1020981320040
Orlova, A.V., Kononov, L., Kimel, B.G., Sivaev, I.B., Bregadze, V.I.: Conjugates of polyhedral boron compounds with carbohydrates. 4. Hydrolytic stability of carborane–lactose conjugates depends on the structure of a spacer between the carborane cage and sugar moiety. Appl. Organomet. Chem. 20, 416–420 (2006). https://doi.org/10.1002/aoc.1082
Kononov, L.O., Malysheva, N.N., Kononova, E.G., Garkusha, O.G.: The first example of synergism in glycosylation. Possible reasons and consequences. Russ. Chem. Bull. 55, 1311–1313 (2006). https://doi.org/10.1007/s11172-006-0419-4
Kononov, L.O., Malysheva, N.N., Kononova, E.G., Orlova, A.V.: Intermolecular hydrogen-bonding pattern of a glycosyl donor: the key to understanding the outcome of sialylation. Eur. J. Org. Chem. 2008, 3251–3255 (2008). https://doi.org/10.1002/ejoc.200800324
Kononov, L.O., Malysheva, N.N., Orlova, A.V.: Stereoselectivity of glycosylation may change during the reaction course: highly α-stereoselective sialylation achieved by supramer approach. Eur. J. Org. Chem. 2009, 611–616 (2009). https://doi.org/10.1002/ejoc.200801017
Kononov, L.O., Malysheva, N.N., Orlova, A.V., Zinin, A.I., Laptinskaya, T.V., Kononova, E.G., Kolotyrkina, N.G.: Concentration dependence of glycosylation outcome: a clue to reproducibility and understanding the reasons behind. Eur. J. Org. Chem. 2012, 1926–1934 (2012). https://doi.org/10.1002/ejoc.201101613
Kononov, L.O.: Modulation of stereoselectivity of glycosylation: a supramer approach. In: Taylor, J.C. (ed.) Advances in Chemistry Research, pp. 143–178. Nova Science Publishers, Inc., Hauppauge (2013)
Orlova, A.V., Andrade, R.R., da Silva, C.O., Zinin, A.I., Kononov, L.O.: Polarimetry as a tool for the study of solutions of chiral solutes. ChemPhysChem 15, 195–207 (2014). https://doi.org/10.1002/cphc.201300894
Orlova, A.V., Zinin, A.I., Kononov, L.O.: Mutarotation in aqueous solutions of D-levoglucosan: a supramer approach. Russ. Chem. Bull. 63, 295–297 (2014). https://doi.org/10.1007/s11172-014-0429-6
Abronina, P.I., Fedina, K.G., Podvalnyy, N.M., Zinin, A.I., Chizhov, A.O., Kondakov, N.N., Torgov, V.I., Kononov, L.O.: The use of O-trifluoroacetyl protection and profound influence of the nature of glycosyl acceptor in benzyl-free arabinofuranosylation. Carbohydr. Res. 396, 25–36 (2014). https://doi.org/10.1016/j.carres.2014.05.017
Kononov, L.O.: Chemical reactivity and solution structure: on the way to a paradigm shift? RSC Adv. 5, 46718–46734 (2015). https://doi.org/10.1039/c4ra17257d
Ahiadorme, D.A., Podvalnyy, N.M., Orlova, A.V., Chizhov, A.O., Kononov, L.O.: Glycosylation of dibutyl phosphate anion with arabinofuranosyl bromide: unusual influence of concentration of the reagents on the ratio of anomeric glycosyl phosphates formed. Russ. Chem. Bull. 65, 2776–2778 (2016)
Kononov, L.O., Fedina, K.G., Orlova, A.V., Kondakov, N.N., Abronina, P.I., Podvalnyy, N.M., Chizhov, A.O.: Bimodal concentration-dependent reactivity pattern of a glycosyl donor: is the solution structure involved? Carbohydr. Res. 437, 28–35 (2017). https://doi.org/10.1016/j.carres.2016.11.009
Orlova, A.V., Tsvetkov, D.E., Kononov, L.O.: Separation of levoglucosan supramers by high performance liquid chromatography. Russ. Chem. Bull. 66, 1712–1715 (2017). https://doi.org/10.1007/s11172-017-1945-y
Orlova, A.V., Laptinskaya, T.V., Bovin, N.V., Kononov, L.O.: Differences in reactivity of N-acetyl- and N,N-diacetylsialyl chlorides, caused by their different supramolecular organization in solutions. Russ. Chem. Bull. 66, 2173–2179 (2017). https://doi.org/10.1007/s11172-017-1999-x
Podvalnyy, N.M., Malysheva, N.N., Panova, M.V., Zinin, A.I., Chizhov, A.O., Orlova, A.V., Kononov, L.O.: Stereoselective sialylation with O-trifluoroacetylated thiosialosides: hydrogen bonding involved? Carbohydr. Res. 451, 12–28 (2017). https://doi.org/10.1016/j.carres.2017.09.002
Stepanova, E.V., Podvalnyy, N.M., Abronina, P.I., Kononov, L.O.: Length matters: one additional methylene group in a reactant is able to affect the reactivity pattern and significantly increase the product yield. Synlett 29, 2043–2045 (2018). https://doi.org/10.1055/s-0037-1610648
Abronina, P.I., Malysheva, N.N., Litvinenko, V.V., Zinin, A.I., Kolotyrkina, N.G., Kononov, L.O.: A ring contraction of 2,3-di-O-silylated thiopyranosides to give thiofuranosides under mildly acidic conditions. Org. Lett. 20, 6051–6054 (2018). https://doi.org/10.1021/acs.orglett.8b02424
Nagornaya, M.O., Orlova, A.V., Stepanova, E.V., Zinin, A.I., Laptinskaya, T.V., Kononov, L.O.: The use of the novel glycosyl acceptor and supramer analysis in the synthesis of sialyl-α(2-3)-galactose building block. Carbohydr. Res. 470, 27–35 (2018). https://doi.org/10.1016/j.carres.2018.10.001
Orlova, A.V., Laptinskaya, T.V., Kononov, L.O.: The first example of detection of mesoscale particles in a solution of a low-molecular-mass compound in dichloromethane. Russ. Chem. Bull. 68, 1462–1464 (2019). https://doi.org/10.1007/s11172-019-2580-6
Myachin, I.V., Orlova, A.V., Kononov, L.O.: Glycosylation in flow: effect of the flow rate and type of the mixer. Russ. Chem. Bull. 68, 2126–2129 (2019). https://doi.org/10.1007/s11172-019-2677-y
Pazynina, G., Tyrtysh, T., Nasonov, V., Belyanchikov, I., Paramonov, A., Malysheva, N., Zinin, A., Kononov, L., Bovin, N.: Divergent strategy for the synthesis of α2-3-linked sialo-oligosaccharide libraries using a Neu5TFA-(α2-3)-Gal building block. Synlett 24, 226–230 (2013). https://doi.org/10.1055/s-0032-1317961
Krickl, S., Buchecker, T., Meyer, A.U., Grillo, I., Touraud, D., Bauduin, P., Konig, B., Pfitzner, A., Kunz, W.: A systematic study of the influence of mesoscale structuring on the kinetics of a chemical reaction. Phys. Chem. Chem. Phys. 19, 23773–23780 (2017). https://doi.org/10.1039/c7cp02134h
Chao, C.-S., Li, C.-W., Chen, M.-C., Chang, S.-S., Mong, K.-K.T.: Low-concentration 1,2-trans β-selective glycosylation strategy and its applications in oligosaccharide synthesis. Chem. Eur. J. 15, 10972–10982 (2009). https://doi.org/10.1002/chem.200901119
Chao, C.S., Lin, C.Y., Mulani, S., Hung, W.C., Mong, K.K.T.: Neighboring-group participation by C-2 ether functions in glycosylations directed by nitrile solvents. Chem. Eur. J. 17, 12193–12202 (2011). https://doi.org/10.1002/chem.201100732
Mong, K.K.T., Yen, Y.F., Hung, W.C., Lai, Y.H., Chen, J.H.: Application of 2-azido-2-deoxythioglycosides for β-glycoside formation and oligosaccharide synthesis. Eur. J. Org. Chem. 2012, 3009–3017 (2012). https://doi.org/10.1002/ejoc.201200173
Yang, F., Zhu, Y., Yu, B.: A dramatic concentration effect on the stereoselectivity of N-glycosylation for the synthesis of 2′-deoxy-β-ribonucleosides. Chem. Commun. 48, 7097–7097 (2012). https://doi.org/10.1039/c2cc33155a
Yasomanee, J.P., Demchenko, A.V.: Effect of remote picolinyl and picoloyl substituents on the stereoselectivity of chemical glycosylation. J. Am. Chem. Soc. 134, 20097–20102 (2012). https://doi.org/10.1021/ja307355n
Visansirikul, S., Yasomanee, J.P., Demchenko, A.V.: Halobenzoyl groups in glycosylation: effect on stereoselectivity and reactivity of glycosyl donors. Russ. Chem. Bull. 64, 1107–1118 (2015). https://doi.org/10.1007/s11172-015-0987-2
Franca, B.A., da Silva, C.O.: Specific rotation of monosaccharides: a global property bringing local information. Phys. Chem. Chem. Phys. 16, 13096–13102 (2014). https://doi.org/10.1039/c4cp01316f
Lagodzinskaya, G.V., Laptinskaya, T.V., Kazakov, A.I.: Supramolecular structuring of aqueous solutions of strong acids: manifestations in light scattering, NMR, and oxidation kinetics. Does liquid have a drop-like nature? 1. Nitric acid. Russ. Chem. Bull. 67, 1838–1850 (2018). https://doi.org/10.1007/s11172-018-2297-y
Lagodzinskaya, G.V., Laptinskaya, T.V., Kazakov, A.I.: Supramolecular structuring of aqueous solutions of strong acids: manifestations in light scattering, NMR, and oxidation kinetics. Does liquid have a drop-like nature? 2. Perchloric acid. Russ. Chem. Bull. 67, 2212–2223 (2018). https://doi.org/10.1007/s11172-018-2358-2
Pecora, R.: Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2, 123–131 (2000). https://doi.org/10.1023/a:1010067107182
Schärtl, W.: Light Scattering from Polymer Solutions and Nanoparticle Dispersions, 1st edn. Springer, Berlin, Heidelberg (2007)
Chu, B.: Laser Light Scattering: Basic Principles and Practice. Academic Press, Boston (1991)
Berne, B.J., Pecora, R.: Dynamic Light Scattering with Applications to Chemistry, Biology, and Physics. Dover, New York (2000)
Provencher, S.W.: A constrained regularization method for inverting data represented by linear algebraic or integral equations. Comput. Phys. Commun. 27, 213–227 (1982). https://doi.org/10.1016/0010-4655(82)90173-4
Provencher, S.W.: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 27, 229–242 (1982). https://doi.org/10.1016/0010-4655(82)90174-6
Teraoka, I.: Polymer Solutions: An Introduction to Physical Properties. Wiley, New York (2002)
Introduction to the calculators in the Zetasizer software (TN120925). Malvern Instruments Limited, Malvern (2014)
Alford, J.R., Kendrick, B.S., Carpenter, J.F., Randolph, T.W.: Measurement of the second osmotic virial coefficient for protein solutions exhibiting monomer–dimer equilibrium. Anal. Biochem. 377, 128–133 (2008). https://doi.org/10.1016/j.ab.2008.03.032
Fried, J.R.: Polymer Science and Technology, 2nd edn. Prentice Hall, Upper Saddle River, NJ (2003)
Wolf, B.A.: Making Flory–Huggins practical: thermodynamics of polymer-containing mixtures. In: Wolf, B.A., Enders, S. (eds.) Polymer Thermodynamics: Liquid Polymer-Containing Mixtures. Adv. Polymer Sci. vol. 238. pp. 1–66. Springer, Berlin, Heidelberg (2011)
Sharipov, A.S., Loukhovitski, B.I., Tsai, C.J., Starik, A.M.: Theoretical evaluation of diffusion coefficients of (Al2O3)n clusters in different bath gases. Eur. Phys. J. D 68, 99 (2014). https://doi.org/10.1140/epjd/e2014-40831-2
Edward, J.T.: Molecular volumes and the Stokes–Einstein equation. J. Chem. Educ. 47, 261–270 (1970). https://doi.org/10.1021/ed047p261
Zhao, Y.H., Abraham, M.H., Zissimos, A.M.: Fast calculation of van der Waals volume as a sum of atomic and bond contributions and its application to drug compounds. J. Org. Chem. 68, 7368–7373 (2003). https://doi.org/10.1021/jo034808o
Krickl, S., Jurko, L., Wolos, K., Touraud, D., Kunz, W.: Surfactant-free microemulsions with cleavable constituents. J. Mol. Liq. 271, 112–117 (2018). https://doi.org/10.1016/j.molliq.2018.08.120
Sedlák, M.: Large-scale supramolecular structure in solutions of low molar mass compounds and mixtures of liquids: 1. Light scattering characterization. J. Phys. Chem. B 110, 4329–4338 (2006). https://doi.org/10.1021/jp0569335
Buchecker, T., Krickl, S., Winkler, R., Grillo, I., Bauduin, P., Touraud, D., Pfitzner, A., Kunz, W.: The impact of the structuring of hydrotropes in water on the mesoscale solubilisation of a third hydrophobic component. Phys. Chem. Chem. Phys. 19, 1806–1816 (2017). https://doi.org/10.1039/c6cp06696h
Krickl, S., Touraud, D., Bauduin, P., Zinn, T., Kunz, W.: Enzyme activity of horseradish peroxidase in surfactant-free microemulsions. J. Colloid Interface Sci. 516, 466–475 (2018). https://doi.org/10.1016/j.jcis.2018.01.077
Hahn, M., Krickl, S., Buchecker, T., Jost, G., Touraud, D., Bauduin, P., Pfitzner, A., Klamt, A., Kunz, W.: Ab initio prediction of structuring/mesoscale inhomogeneities in surfactant-free microemulsions and hydrogen-bonding-free microemulsion. Phys. Chem. Chem. Phys. 21, 8054–8066 (2019). https://doi.org/10.1039/c8cp07544a
Troncoso, J., Zemánková, K., Jover, A.: Dynamic light scattering study of aggregation in aqueous solutions of five amphiphiles. J. Mol. Liq. 241, 525–529 (2017). https://doi.org/10.1016/j.molliq.2017.06.022
Sedlák, M., Rak, D.: On the origin of mesoscale structures in aqueous solutions of tertiary butyl alcohol: the mystery resolved. J. Phys. Chem. B 118, 2726–2737 (2014). https://doi.org/10.1021/jp500953m
Lagodzinskaya, G.V., Laptinskaya, T.V., Kazakov, A.I., Kurochkina, L.S., Manelis, G.B.: Slow large-scale supramolecular structuring as a cause of kinetic anomalies in the liquid-phase oxidation with nitric acid. Russ. Chem. Bull. 65, 984–992 (2016). https://doi.org/10.1007/s11172-016-1401-4
Svard, M., Renuka Devi, K., Khamar, D., Mealey, D., Cheuk, D., Zeglinski, J., Rasmuson, A.C.: Solute clustering in undersaturated solutions – systematic dependence on time, temperature and concentration. Phys. Chem. Chem. Phys. 20, 15550–15559 (2018). https://doi.org/10.1039/c8cp01509k
Rak, D., Sedlák, M.: On the mesoscale solubility in liquid solutions and mixtures. J. Phys. Chem. B 123, 1365–1374 (2019). https://doi.org/10.1021/acs.jpcb.8b10638
Rak, D., Ovadová, M., Sedlák, M.: (Non)existence of bulk nanobubbles: the role of ultrasonic cavitation and organic solutes in water. J. Phys. Chem. Lett. 10, 4215–4221 (2019). https://doi.org/10.1021/acs.jpclett.9b01402
Zemb, T., Kunz, W.: Weak aggregation: state of the art, expectations and open questions. Curr. Opin. Colloid Interface Sci. 22, 113–119 (2016). https://doi.org/10.1016/j.cocis.2016.04.002
Enami, S., Ishizuka, S., Colussi, A.J.: Chemical signatures of surface microheterogeneity on liquid mixtures. J. Chem. Phys. 150, 024702 (2019). https://doi.org/10.1063/1.5055684
Qiu, J., Ishizuka, S., Tonokura, K., Colussi, A.J., Enami, S.: Water dramatically accelerates the decomposition of α-hydroxyalkyl-hydroperoxides in aerosol particles. J. Phys. Chem. Lett. 10, 5748–5755 (2019). https://doi.org/10.1021/acs.jpclett.9b01953
Acknowledgements
This work was financially supported by the Russian Science Foundation (Project No. 16-13-10244-P).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Orlova, A.V., Laptinskaya, T.V., Malysheva, N.N. et al. Light Scattering in Non-aqueous Solutions of Low-Molecular-Mass Compounds: Application for Supramer Analysis of Reaction Solutions. J Solution Chem 49, 629–644 (2020). https://doi.org/10.1007/s10953-020-00977-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00977-1