Abstract
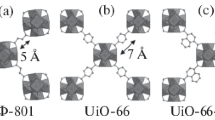
Zirconium metal-organic frameworks UIO-66(H2ADC) was synthesized with 9,10-anthracenedicarboxylic acid as ligand by the simple solution method (SS)and solvothermal reaction (S). The influence of different reaction conditions on the structure and properties of UIO-66(H2ADC) were investigated. The structures of samples UIO-66(H2ADC) were characterized by the powder X-ray diffraction, infrared spectroscopy and nitrogen sorption technique. UIO-66(H2ADC) and UIO-66 had the same face-centered cubic topology. When the feed ratio was 1.2:1 (metal:organic linker), the reaction temperature was 45 °C and reaction time was 12 h by the simple solution method (which was named UIO-66(H2ADC)-SS), UIO-66(H2ADC)-SS had the best crystallinity and BET specific surface area (432 m2/g) with average pore size of around 4.38 nm. And when at reaction condition was temperature of 85 °C, the feed ratio of 0.61:1(metal:organic linker) and time of 24 h by solvothermal reaction (denoted as UIO-66(H2ADC)-S), UIO-66(H2ADC)-S had the biggest BET specific surface area (772 m2/g) and had smaller average size of around 2.73 nm. The hydrogen storage properties of different samples were determined by hydrogen storage analyzer. UIO-66(H2ADC)-S and UIO-66(H2ADC)-SS hydrogen uptake capacity were 29.2 and 10.9 mg/g at 298 K, 5 MPa, respectively. When considering of unit the specific surface area adsorption UIO-66(H2ADC)-S and UIO-66(H2ADC)-SS were 0.0378 and 0.0252 mg/m2, but UIO-66 was 0.0233 mg/m2 ,which shown UIO-66(H2ADC) have good hydrogen uptake capacity. Interaction energy of UIO-66(H2ADC) and hydrogen was − 5.73 kJ/mol.





Similar content being viewed by others
References
S. Chen, J. Liu, Z. Li, H. Wang, X. Wang, Y. Xu, Int. J. Hydrog. Energy 42, 36 (2017)
J.L. Rowsell, O.M. Yaghi, Angew. Chem. Int. Ed. Engl. 44, 30 (2005)
J. Goldsmith, A.G. Wong-Foy, M.J. Cafarella, D.J. Siegel, Chem. Mater. 25, 16 (2013)
M. Rubio-Martinez, C. Avci-Camur, A.W. Thornton, I. Imaz, D. Maspoch, M.R. Hill, Chem. Soc. Rev. 46, 11 (2017)
O.M. Yaghi, M. O’Keeffe, N.W. Ockwig, H.K. Chae, M. Eddaoudi, J. Kim, Nature 423, 6941 (2003)
K.K. Tanabe, S.M. Cohen, Chem. Soc. Rev. 40, 2 (2011)
J.H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga, K.P. Lillerud, J. Am. Chem. Soc. 130, 42 (2008)
F. Yang, H. Huang, X. Wang, F. Li, Y. Gong, C. Zhong, J.-R. Li, Cryst. Growth Des. 15, 12 (2015)
F. Ragon, B. Campo, Q. Yang, C. Martineau, A.D. Wiersum, A. Lago, V. Guillerm, C. Hemsley, J.F. Eubank, M. Vishnuvarthan, F. Taulelle, P. Horcajada, A. Vimont, P.L. Llewellyn, M. Daturi, S. Devautour-Vinot, G. Maurin, C. Serre, T. Devic, G. Clet, J. Mater. Chem. A 3, 7 (2015)
M. Kandiah, M.H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E.A. Quadrelli, F. Bonino, K.P. Lillerud, Chem. Mater. 22, 24 (2010)
M. Yoon, D. Moon, Microporous Mesoporous Mater. 215, 116–122 (2015)
Q. Zhao, W. Yuan, J. Liang, J. Li, Int. J. Hydrog. Energy 38, 29 (2013)
J. Ren, N.M. Musyoka, H.W. Langmi, B.C. North, M. Mathe, X. Kang, S. Liao, Int. J. Hydrog. Energy 40, 33 (2015)
B. Wang, X.L. Lv, D. Feng, L.H. Xie, J. Zhang, M. Li, Y. Xie, J.R. Li, H.C. Zhou, J. Am. Chem. Soc. 138, 19 (2016)
U. Herrmann, B. Tiimmler, G. Maass, P.K.T. Mew, F. Vogtle, Biochemistry 23, 18 (1984)
S.-B. Xiao, S.-S. Chen, J. Liu, Z. Lin, F.-J. Zhang, X.-B. Wang, W.-C. Oh, J. Korean Ceram. Soc. 53, 2 (2016)
Y. Bai, Y. Dou, L.H. Xie, W. Rutledge, J.R. Li, H.C. Zhou, Chem. Soc. Rev. 45, 8 (2016)
S.S. Han, W.Q. Deng, W.A. Goddard 3rd, Angew. Chem. Int. Ed. Engl. 46, 33 (2007)
Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 21171004), and the Science and Technology Project of Anhui Province (No. 1604a0802113), and Anhui Province Academic Technology Leader Training Funded Projects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Xiao, S., Liu, J. et al. Synthesis and hydrogen storage properties of zirconium metal-organic frameworks UIO-66(H2ADC) with 9,10-anthracenedicarboxylic acid as ligand. J Porous Mater 25, 1783–1788 (2018). https://doi.org/10.1007/s10934-018-0591-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0591-6