Abstract
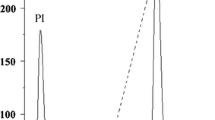
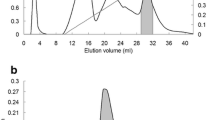
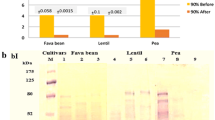
The Abelmoschus esculentus (Malvaceae) plant originated in Africa and has spread across a number of tropic countries, including northeastern Brazil. The plant has been used to treat various disorders, such as cancer, microbial infections, hypoglycemia, constipation, urine retention and inflammation. The lectin of A. esculentus (AEL) was isolated by precipitation with ammonium sulfate at a saturation level of 30/60 and purified by ion exchange chromatography (Sephacel-DEAE). The electrophoresis (SDS-PAGE) profile of the AEL showed two protein bands of apparent molecular mass of approximately 15.0 and 21.0 kDa. The homogenity of the protein was confirmed by electrospray mass spectrometry (ESI-MS), which revealed the presence of a 10.29-kDa monomer and a 20.58-kDa dimer. The AEL exhibits agglutinating activity against rabbit (74.41 UH/mP) and human type ABO erythrocytes (21.00 UH/mP). This activity does not require the presence of divalent cations and is specifically inhibited by lactose, fructose and mannose. The intravenous treatment with 0.01, 0.1 and 1 mg/kg of AEL inhibited the paw edema elicited by carrageenan by approximately 15, 22 and 44 %, respectively, but not that induced by dextran. In addition, treatment with 0.1, 1 and 10 mg/kg of AEL also inhibited the abdominal writhing induced by acetic acid by approximately 52, 57 and 69 %, respectively. In conclusion, AEL is a new lectin with a molecular mass of 20.0 kDa, which is -composed of a 10.291-Da monomer and a 20.582-kDa dimer, that exhibits anti-inflammatory, antinociceptive and hemagglutinating activities. In addition, the lectin hemagglutinating property is both metallo-independent and associated with the lectin domain.




Similar content being viewed by others
References
Alencar NM, Assreuy AMS, Havt A, Benevides RG, Moura TR, Sousa RB, Ribeiro RA, Cunha FQ, Cavada BS (2007) Naunyn Schmiedebergs Arch Pharmacol 374:275–282
Assreuy AM, Fontenele SR, Pires AF, Fernandes DC, Rodrigues NV, Bezerra EH, Moura TR, Nascimento KS, Cavada BS (2009) Naunyn Schmiedebergs Arch Pharmacol 380:509–521
Assreuy AM, Martins GJ, Moreira EE, Brito GA, Cavada BS, Ribeiro RA, Flores CA (1999) J Urol 161:1988–1993
Assreuy AM, Shibuya MD, Martins GJ, Souza ML, Cavada BS, Moreira RA, Oliveira JT, Ribeiro RA, Flores CA (1997) Mediat Inflamm 6:201–210
Caetano N, Saraiva A, Pereira R, Carvalho D, Pimentel MCB, Maia MBS (2002) Rev Bras Farmacogn 12:132–135
Cavada BS, Barbosa T, Arruda S, Grangeiro TB, Barral Netto M (2001) Curr Protein Pept 2:123–135
Collier HO, Dinneen LC, Johnson CA, Schneider C (1968) Br J Pharmacol 32:295
da Silva ALC, Horta ACG, Moreira RA (2001) Rev Bras Fisiol Veg 13:262–269
Delatorre P, Rocha BA, Simões RC, Pereira-Júnior FN, Silva HC, Bezerra EH, Bezerra MJ, Marinho ES, Gadelha CA, Santi-Gadelha T, Farias DL, Assreuy AM, Marques-Domingos GF, Nagano CS, Cavada BS (2011) Appl Biochem Biotechnol 164:741–754
Delatorre P, Rocha BAM, Souza EP, Oliveira TM, Bezerra GA, Moreno FB, Freitas BT, Santi-Gadelha T, Sampaio AH, Azevedo-Júnior WF, Cavada BS (2007) BMC Struct Biol 7:1–9
Dirosa M, Giroud JP, Willoughby DA (1971) J Path 104:15–29
Edelman GM, Wang JL (1978) J Biol Chem 253:3016–3022
Ferrige AG, Seddon MJ, Green BN, Jarvis SA, Skilling J (1992) Rapid Commun Mass Spectrom 6:707–711
Figueiredo JG, Silveira BF, Beserra IG, Teixeira CS, Luz PB, Bezerra EHS, Mota MRL, Assreuy AMS, Queiroz CF, Cavada BS, Alencar NMN (2009) Naunyn Schmiedebergs Arch Pharmacol 380:407–414
Gadelha CAA, Moreno FBMB, Santi-Gadelha T, Cajazeiras JB, Rocha BAM, Assreuy AM, Mota MRL, Pinto NV, Meireles AVP, Borges JC, Freitas BT, Canduri F, Souza EP, Delatorre P, Criddle DN, Azevedo-Júnior WF, Cavada BS (2005) J Struct Biol 152:185–194
Gurbuz I, Ustun O, Yesilada E, Sezik E, Akyurek N (2003) J Ethnopharmacol 83:241–244
Holanda FR, Sousa ANC, Assreuy AM, Cardoso JHL, Pires AF, Nascimento KS, Teixeira CS, Cavada BS, Santos CF (2009) Protein Pept Lett 16:1088–1092
Koster R, Anderson M, Beer EJ (1959) Fed Proc 18:412–416
Kumar R, Patil MB, Patil SR, Paschapur MS (2009) Int J Pharm Technol Res 1:658–665
Laemmli UK (1970) Nature 227:680–685
Landucci ECT, Antunes E, Donato JL, Faro R, Hyslop S, Marangoni S, Oliveira B, Cirino G, Nucci G (1995) Brit J Pharmacol 114:578–583
Lo TN, Almeida AP, Beaven MA (1982) J Pharmacol Exp Ther 221:261–267
Masnikosa R, Nikolić AJ, Nedić O (2008) J Serbian Chem Soc 73:793–804
Mota MRL, Criddle DN, Alencar NMN, Gomes RC, Meireles AVP, Santi-Gadelha T, Gadelha CAA, Oliveira CC, Benevides RG, Cavada BS, Assreuy AMS (2006) Naunyn Schmiedebergs Arch Pharmacol 374:1–10
Pal S, Chakrborty SK, Banerjee A, Mukharji B (1968) Indian J Med Res 56:445–455
Peumans WJ, Barre A, Hao Q, Rougé P, Van Damme EJM (2000) Trends Glycosci Glycotechnol 12:83–101
Rangel TB, Assreuy AM, Pires AF, Carvalho AU, Benevides RG, Simões RC, Silva HC, Bezerra MJ, Nascimento AS, Nascimento KS, Nagano CS, Sampaio AH, Delatorre P, Rocha BA, Fernandes PM, Cavada BS (2011) Molecules 20:5087–5103
Rates SMK (2001) Toxicon 39:603–613
Santi-Gadelha T, Gadelha CAA, Aragão KS, Oliveira CC, Mota MRL, Gomes RC, Pires AF, Toyama MH, Toyama DO, Alencar NMN, Criddle DN, Cavada BS, Assreuy AMS (2006) Biochem Biophys Res Commun 350:1050–1055
Santi-Gadelha T, Rocha BAM, Oliveira CC, Aragão KS, Marinho ES, Gadelha CAA, Toyama MH, Pinto VPT, Nagano CS, Delatorre P, Martins JL, Galvani FN, Sampaio AH, Debray H, Cavada BS (2008) Appl Biochem Biotechnol 150:97–111
Scopes RK (1994) Protein purification: principles and practices. Springer Verlag
Sharon N, Lis H (2003) Lectins, 2nd edn. Kluwer, Dordrecht/Netherlands
Silva HC, Bari AU, Pereira-Júnior FN, Simões RC, Barroso-Neto IL, Nobre CB, Pereira MG, Nascimento KS, Rocha BA, Delatorre P, Nagano CS, Assreuy AM, Cavada BS (2011) Protein Pept Lett 18:396–402
Tomoda M, Shiniza N, Oshima Y, Takahashi M, Murakami M, Hikino H (1987) Planta Med 53:8–12
Vasconcelos SMM, Lima SR, Soares PM, Assreuy AMS, Sousa FCF, Lobato RFG, Vasconcelos GS, Santi-Gadelha T, Bezerra EHS, Cavada BS, Patrocinio MCA (2009) Epilepsy Behav 15:291–293
Veloso HP, Goes Filho L (1982) Projeto RADAM Brasil. Boletim Técnico, Série Vegetação 1:3–79
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). B.S. Cavada, P. Delatorre and A.M.S. Assreuy are senior investigators of CNPq/Brazil.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Sousa Ferreira Soares, G., Assreuy, A.M.S., de Almeida Gadelha, C.A. et al. Purification and Biological Activities of Abelmoschus esculentus Seed Lectin. Protein J 31, 674–680 (2012). https://doi.org/10.1007/s10930-012-9447-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-012-9447-0