Abstract
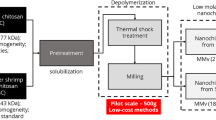
The many applications of the chitosan biopolymers in the areas of nanotechnology and nanomaterials have led to the need to develop novel techniques suitable for the production of nanochitosan. At present these typically involve the use of chemical agents to form chitosan nanoparticles. Physical, chemical, and enzymatic methods have been described for the depolymerizatiton of chitosan. In this work, evaluation was made of the efficiency of a combination of physical methods, including milling of the raw material and drying of the final chitosan, in order to obtain nanochitosan with low molar mass. The results revealed the effectiveness of the combination of milling the raw material under controlled conditions for 4.5 h and drying of the chitosan by thermal shock, which provided depolymerization of up to 10× and resulted in chitosan with Mv less than 21 kDa and hydrodynamic diameter below 30 nm.





Similar content being viewed by others
References
Arantes MK, Kugelmeier CL, Cardozo-Filho L et al (2014) Influence of the drying route on the depolymerization and properties of chitosan. Polym Eng Sci 55:1969–1976. https://doi.org/10.1002/pen
Muanprasat C, Chatsudthipong V (2017) Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther 170:80–97. https://doi.org/10.1016/j.pharmthera.2016.10.013
Kaya M, Asan-Ozusaglam M, Erdogan S (2016) Comparison of antimicrobial activities of newly obtained low molecular weight scorpion chitosan and medium molecular weight commercial chitosan. J Biosci Bioeng 121:678–684. https://doi.org/10.1016/j.jbiosc.2015.11.005
Tan W, Zhang J, Luan F et al (2017) Design, synthesis of novel chitosan derivatives bearing quaternary phosphonium salts and evaluation of antifungal activity. Int J Biol Macromol 102:704–711. https://doi.org/10.1016/j.ijbiomac.2017.04.073
Gopal GRY, Nandakumar KSLC. (2015) Chitosan nanoparticles for generating novel systems for better applications: a review. J Mol Genet Med s4:005. https://doi.org/10.4172/1747-0862.S4-005
Elgadir MA, Uddin MS, Ferdosh S et al (2015) Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: a review. J Food Drug Anal 23:619–629. https://doi.org/10.1016/j.jfda.2014.10.008
Mura S, Corrias F, Stara G et al (2011) Innovative composite films of chitosan, methylcellulose, and nanoparticles. J Food Sci 76:54–60. https://doi.org/10.1111/j.1750-3841.2011.02295.x
Gupta VK, Fakhri A, Agarwal S, Azad M (2017) Synthesis and characterization of Ag2S decorated chitosan nanocomposites and chitosan nanofibers for removal of Lincosamides antibiotic. Int J Biol Macromol 103:1–7. https://doi.org/10.1016/j.ijbiomac.2017.05.018
Tamer TM, Hassan MA, Omer AM et al (2017) Antibacterial and antioxidative activity of O-amine functionalized chitosan. Carbohydr Polym 169:441–450. https://doi.org/10.1016/j.carbpol.2017.04.027
Sivakami MS, Gomathi T, Venkatesan J et al (2013) Preparation and characterization of nano chitosan for treatment wastewaters. Int J Biol Macromol 57:204–212. https://doi.org/10.1016/j.ijbiomac.2013.03.005
Seyedi SM, Anvaripour B, Motavassel M, Jadidi N (2013) Comparative cadmium adsorption from water by nanochitosan and chitosan. Int J Eng Innov Technol 5:145–148
Farid MS, Shariati A, Badakhshan A, Anvaripour B (2013) Using nano-chitosan for harvesting microalga Nannochloropsis sp. Bioresour Technol 131:555–559. https://doi.org/10.1016/j.biortech.2013.01.058
Davis SP (2011) CHitosan: manufacture, properties, and usage. Nova Science Publishers, Inc, New York
Fan W, Yan W, Xu Z, Ni H (2012) Biointerfaces Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces 90:21–27. https://doi.org/10.1016/j.colsurfb.2011.09.042
Dmour I, Taha MO (2017) Novel nanoparticles based on chitosan-dicarboxylate conjugates via tandem ionotropic/covalent crosslinking with tripolyphosphate and subsequent evaluation as drug delivery vehicles (B). Int J Pharm 529:15–31. https://doi.org/10.1016/j.ijpharm.2017.06.061
Alves HJ, Furman M, Kugelmeier CL et al (2017) Effect of shrimp shells milling on the molar mass of chitosan. Polímeros 27:41–47
Delezuk JA, de M, Cardoso, Domard MB, Campana-Filho A SP (2011) Ultrasound-assisted deacetylation of beta-chitin: Influence of processing parameters. Polym Int 60:903–909. https://doi.org/10.1002/pi.3037
Mohammadi A, Hashemi M, Masoud Hosseini S (2016) Effect of chitosan molecular weight as micro and nanoparticles on antibacterial activity against some soft rot pathogenic bacteria. LWT - Food Sci Technol 71:347–355. https://doi.org/10.1016/j.lwt.2016.04.010
Vasilieva T, Sigarev A, Kosyakov D et al (2017) Formation of low molecular weight oligomers from chitin and chitosan stimulated by plasma-assisted processes. Carbohydr Polym 163:54–61. https://doi.org/10.1016/j.carbpol.2017.01.026
No HK, Nah JW, Meyers SP (2002) Effect of time/temperature treatment parameters on depolymerization of chitosan. J Appl Polym Sci 87:1890–1894
San Juan A, Montembault A, Gillet D et al (2012) Degradation of chitosan-based materials after different sterilization treatments. In: 6th EEIGM international conference on advanced materials research, pp 1–5
Yue W (2014) Prevention of browning of depolymerized chitosan obtained by gamma irradiation. Carbohydr Polym 101:857–863. https://doi.org/10.1016/j.carbpol.2013.10.011
Jung J, Zhao Y (2011) Characteristics of deacetylation and depolymerization of β-chitin from jumbo squid (Dosidicus gigas) pens. Carbohydr Res 346:1876–1884. https://doi.org/10.1016/j.carres.2011.05.021
Pan AD, Zeng HY, Foua GB et al (2016) Enzymolysis of chitosan by papain and its kinetics. Carbohydr Polym 135:199–206. https://doi.org/10.1016/j.carbpol.2015.08.052
Dziril M, Grib H, Laribi-Habchi H et al (2015) Chitin oligomers and monomers production by coupling γ radiation and enzymatic hydrolysis. J Ind Eng Chem 26:396–401. https://doi.org/10.1016/j.jiec.2014.12.015
Tsao CT, Chang CH, Lin YY et al (2011) Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation t. Carbohydr Res 346:94–102. https://doi.org/10.1016/j.carres.2010.10.010
Tian F, Liu Y, Hu K, Zhao B (2004) Study of the depolymerization behavior of chitosan by hydrogen peroxide. Carbohydr Polym 57:31–37. https://doi.org/10.1016/j.carbpol.2004.03.016
Alves HJ, Arantes MK, Muniz GIB, Ellendersen LSN (2017) Patente: Privilégio de Inovação. Número do registro: BR102017022250: “Processamento de exoesqueleto de crustáceos para obtenção de nanoquitosana”, Instituição de registro: INPI - Instituto Nacional da Propriedade Industrial, Depósito: 16/10/2017, Brasil, 2017
Kasaai MR (2007) Calculation of Mark–Houwink–Sakurada (MHS) equation viscometric constants for chitosan in any solvent—temperature system using experimental reported viscometric constants data. Carbohydr Polym 68:477–488. https://doi.org/10.1016/j.carbpol.2006.11.006
Santos ZM, Caroni ALPF., Pereira MR et al (2009) Determination of deacetylation degree of chitosan: a comparison between conductometric titration and CHN elemental analysis. Carbohydr Res 344:2591–2595. https://doi.org/10.1016/j.carres.2009.08.030
Berne BJ, Pecora R (2000) Dynamic light scattering: with applications to chemistry, biology, and physics. Wiley, New York
Pecora R (2000) Dynamic light scattering measurement of nanometer particles in liquids. J Nanoparticle Res 2:123–131
Mao S, Shuai X, Unger F et al (2004) The depolymerization of chitosan: effects on physicochemical and biological properties. Int J Pharm 281:45–54. https://doi.org/10.1016/j.ijpharm.2004.05.019
Choi W-S, Ahn K-J, Lee D-W et al (2002) Preparation of chitosan oligoment by irradiation. Plymer Degrad Stab 78:533–538
Jia Z, Shen D (2002) Effect of reaction temperature and reaction time on the preparation of low-molecular-weight chitosan using phosphoric acid. Carbohydr Polym 49:393–396. https://doi.org/10.1016/S0144-8617(02)00026-7
Czechowska-Biskup R, Rokita B, Lotfy S et al (2005) Degradation of chitosan and starch by 360-kHz ultrasound. Carbohydr Polym 60:175–184. https://doi.org/10.1016/j.carbpol.2004.12.001
Zhou HY, Chen XG, Kong M et al (2008) Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr Polym 73:265–273. https://doi.org/10.1016/j.carbpol.2007.11.026
Zhang W, Zhang J, Jiang Q, Xia W (2012) Physicochemical and structural characteristics of chitosan nanopowders prepared by ultrafine milling. Carbohydr Polym 87:309–313. https://doi.org/10.1016/j.carbpol.2011.07.057
Zainol I, Akil HM, Mastor A (2009) Effect of γ-irradiation on the physical and mechanical properties of chitosan powder. Mater Sci Eng C 29:292–297. https://doi.org/10.1016/j.msec.2008.06.026
Kasaai MR, Arul J, Charlet G (2008) Fragmentation of chitosan by ultrasonic irradiation. Ultrason Sonochem 15:1001–1008. https://doi.org/10.1016/j.ultsonch.2008.04.005
Luo WB, Han Z, Zeng XA et al (2010) Study on the degradation of chitosan by pulsed electric fields treatment. Innov Food Sci Emerg Technol 11:587–591. https://doi.org/10.1016/j.ifset.2010.04.002
Zhang W, Zhang J, Xia W (2014) Effect of ball-milling treatment on physicochemical and structural properties of chitosan. Int J Food Prop 17:26–37. https://doi.org/10.1080/10942912.2011.608175
Lim LY, Khor E, Ling CE (1999) Effects of dry heat and saturated steam on the physical properties of chitosan. J Biomed Mater Res 48:111–116
Tsaih ML, Chen RH (2003) The effect of reaction time and temperature during heterogenous alkali deacetylation on degree of deacetylation and molecular weight of resulting chitosan. J Appl Polym Sci 88:2917–2923. https://doi.org/10.1002/app.11986
Gomes LP, Andrade CT, Silva JT et al (2014) Green synthesis and physical and chemical characterization of chitosans with a high degree of deacetylation, produced by a binary enzyme system. J Life Sci 8:276–282
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alves, H.J., Vieceli, M., Alves, C. et al. Chitosan Depolymerization and Nanochitosan Production Using a Single Physical Procedure. J Polym Environ 26, 3913–3923 (2018). https://doi.org/10.1007/s10924-018-1267-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-018-1267-7