Abstract
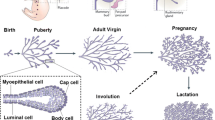
We propose a new scenario for mammary evolution based on comparative review of early mammary development among mammals. Mammary development proceeds through homologous phases across taxa, but evolutionary modifications in early development produce different final morphologies. In monotremes, the mammary placode spreads out to form a plate-like mammary bulb from which more than 100 primary sprouts descend into mesenchyme. At their distal ends, secondary sprouts develop, including pilosebaceous anlagen, resulting in a mature structure in which mammary lobules and sebaceous glands empty into the infundibula of hair follicles; these structural triads (mammolobular-pilo-sebaceous units or MPSUs) represent an ancestral condition. In marsupials a flask-like mammary bulb elongates as a sprout, but then hollows out; its secondary sprouts include hair and sebaceous anlagen (MPSUs), but the hairs are shed during nipple formation. In some eutherians (cat, horse, human) MPSUs form at the distal ends of primary sprouts; pilosebaceous components either regress or develop into mature structures. We propose that a preexisting structural triad (the apocrine-pilo-sebaceous unit) was incorporated into the evolving mammary structure, and coupled to additional developmental processes that form the mammary line, placode, bulb and primary sprout. In this scenario only mammary ductal trees and secretory tissue derive from ancestral apocrine-like glands. The mammary gland appears to have coopted signaling pathways and genes for secretory products from even earlier integumentary structures, such as odontode (tooth-like) or odontode-derived structures. We speculate that modifications in signal use (such as PTHrP and BMP4) may contribute to taxonomic differences in MPSU development.







Similar content being viewed by others
Abbreviations
- APSU:
-
Apo-pilo-sebaceous unit
- BMP:
-
Bone morphogenic protein
- CRL:
-
Crown-rump length
- EDA:
-
Ectodysplasin
- FGF:
-
Fibroblast growth factor
- Ihh:
-
Indian hedgehog
- MB:
-
Mammary bulb
- MG:
-
Mammary gland
- ML:
-
Mammary line
- MP:
-
Mammary placode
- MPSU:
-
Mammolobular-pilo-sebaceous unit
- mya:
-
Million years ago
- PS:
-
Primary sprout
- PTHrP:
-
Parathyroid hormone-related protein
- SG:
-
Sebaceous gland
- Shh:
-
Sonic hedgehog
- SS:
-
Secondary sprout
References
Oftedal OT. The evolution of milk secretion and its ancient origins. Animal. 2012;6:355–68.
Oftedal OT. Origin and evolution of the major constituents of milk. In: McSweeney PLH, Fox PF, editors. Advanced dairy chemistry: volume 1A: proteins. Basic aspects. Boston: Springer US; 2013. p. 1–42.
Benton MJ. Vertebrate paleontology. 3rd ed. Malden: Blackwell Publishing; 2005.
Kemp TS. The origin and evolution of mammals. New York: Oxford University Press; 2005.
Luo Z-X, Yuan CX, Meng Q-J, Ji Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature. 2011;476:442–5.
Clack JA. Gaining ground. The origin and evolution of tetrapods. 2nd ed. Bloomington: Indiana University Press; 2012.
Wells KD. The ecology and behavior of amphibians. Chicago: University of Chicago Press; 2007.
Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia. 2002;7(3):253–66.
Oftedal OT. The mammary gland and its origin during synapsid evolution. J Mammary Gland Biol Neoplasia. 2002;7(3):225–52.
Dhouailly D. A new scenario for the evolutionary origin of hair, feather, and avian scales. J Anat. 2009;214(4):587–606.
Lemay DG, Lynn DJ, Martin WF, Neville MC, Casey TM, Rincon G, et al. The bovine lactation genome: insights into the evolution of mammalian milk. Genome Biol. 2009;10(4):R43. doi:10.1186/gb-2009-10-4-r43.
Chapman RE. Hair, wool, quill, nail, claw, hoof, and horn. In: Bereiter-Hahn J, Matolstoy AG, Richards KS, editors. Biology of the integument. 2. Vertebrates. Berlin: Springer Verlag; 1986. p. 293–317.
Craigmyle MBL. The apocrine glands and the breast. New York: John Wiley and Sons; 1984.
Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:1–17.
Vorherr H. The breast. Morphology, physiology and lactation. New York: Academic; 1974.
Anderson R. Embryonic and fetal development of the mammary apparatus. In: Larson B, editor. Lactation: a comprehensive treatise. Volume IV. The mammary gland/ human lactation/ milk synthesis. New York: Academic; 1978. p. 3–40.
Griffiths M. Biology of the monotremes. New York: Academic; 1978.
Bresslau E. Die Entwickelung des Mammarapparates der Monotremen, Marsupialier und einiger Placentalier. Ein Beitrag zur Phylogenie der Saugethiere. I. Entwickelung und Ursprung des Mammarapparates von Echidna. Denskschr Med-Naturwiss Gesellsch Jena. 1907;7(5):455–518. plates 28–30.
Griffiths M, Elliott MA, Leckie RMC, Schoefl GI. Observations of the comparative anatomy and ultrastructure of mammary glands and on the fatty acids of the triglycerides in platypus and echidna milk fats. J Zool. 1973;169:255–79.
Nilsson M, Churakov G, Sommer M, Tran N, Zemann A, Brosius J, et al. Tracking marsupial evolution using archaic genomic retroposon insertions. PLoS Biol. 2010;8:e1000436. doi:10.1371/journal.pbio.1000436.
Hughes R, Hall L. Structural adaptations of the newborn marsupial. In: Tyndale-Biscoe CH, Janssens P, editors. The developing marsupial. Models for biomedical research. Berlin: Springer Verlag; 1988. p. 8–27.
Bresslau E. Die Entwickelung des Mammarapparates der Monotremen, Marsupialier und einiger Placentalier. III. Entwickelung des Mammarapparates der Marsupialier, Insectivoren, Nagathiere, Carnivoren und Wiederkäuer. Denskschr Med-Naturwiss Gesellsch Jena. 1912;7(5):647–874. plates 37–46.
Tyndale-Biscoe H, Renfree M. Reproductive physiology of marsupials. Cambridge: Cambridge University Press; 1987.
Green B, Merchant J. The composition of marsupial milk. In: Tyndale-Biscoe CH, Janssens PA, editors. The developing marsupial. Models for biomedical research. Berlin: Springer Verlag; 1988. p. 41–54.
Bresslau E. Beiträge zur Entwicklungsgeschichte der Mammarorgane bei den Beutelthieren. Z Morphol Anthropol. 1902;4:261–317.
Griffiths M, McIntosh D, Leckie RMC. The mammary glands of the red kangaroo with observations on the fatty acid components of the milk triglycerides. J Zool. 1972;166:265–75.
Cowin P, Wysolmerski J. Molecular mechanisms guiding embryonic mammary gland development. Cold Spring Harb Perspect Biol. 2011;2:a003251. doi:10.1101/cshperspect.a003251.
Turner CW. The mammary gland. I. The anatomy of the udder of cattle and domestic animals. Columbia: Lucas Brothers; 1952.
Raynaud A. Morphogenesis of the mammary gland. In: Kon S, Cowie A, editors. Milk: the mammary gland and its secretion. New York: Academic; 1961. p. 3–46.
Rowson AR, Daniels KM, Ellis SE, Hovey RC. Growth and development of the mammary glands of livestock: a veritable barnyard of opportunities. Semin Cell Dev Biol. 2012;23:557–66.
Uehlinger P. Studien zur Entwicklung der Milchdrüse des Pferdes. 11. Beitrag zum Bau und zur Entwicklung von Hautorganen bei Saugetieren. Inaugural-Dissertation zur Erlangung der Doctor-Würde. Zurich: University of Zurich; 1922.
Profé O. Beiträge zur Ontogenie und Phylogenie der Mammarorgane. Anat Hefte. 1899;11:245–86.
Rein G. Untersuchungen über die embryonale Enwicklungsgeschichte der Milchdrüse. II. Vergleichend-anatomische Ergebnisse und Schlussresultate. Arch Mikrosk Anat. 1882;21:678–94. plate 30.
Hamburger C. Studien zur Entwickelung der Mammarorgane. I. Die Zitze von Pferd und Esel. Anat Anz. 1900;18:16–26.
Brouha M. Recherches sur les diverses phases du développement et de l’activité de la mamelle. Arch Biol. 1905;21:459–603. plates 18–20.
Dabelow A. Die Milchdrüse. In: von Möllendorff W, Bargmann W, editors. Handbuch der mikroskopischen Anatomie des Menschen. Dritter Band, Haut und Sinnesorgane. Dritter Teil, die Haut die Milchdrüse. Berlin: Springer Verlag; 1957. p. 277–485.
Jolicoeur F. Intrauterine breast development and the mammary myoepithelial lineage. J Mammary Gland Biol Neoplasia. 2005;10:199–210.
Ba G, Stein T. Human breast development. Semin Cell Dev Biol. 2012;23:567–73.
Howard BA, Gusterson BA. Human breast development. J Mammary Gland Biol Neoplasia. 2000;5:119–37.
Russo J, Russo IH. Development of the human mammary gland. In: Neville MC, Daniel CW, editors. The mammary gland. Development, regulation and function. New York: Plenum Press; 1987. p. 67–93.
Hassiotou F, Geddes D. Anatomy of the human mammary gland: current status of knowledge. Clin Anat. 2013;26:29–48.
Hughes ESR. Development of the mammary gland. Ann R Coll Surg Engl. 1950;6:99–119.
Broman I. Normale und abnorme Entwicklung des Menschen. Wiesbaden: Verlag von JF Bergmann; 1911.
Cooper AP. On the anatomy of the breast. London: Longman, Orme, Green, Brown and Longmans; 1840.
Love SM, Barsky SH. Anatomy of the nipple and breast ducts revisited. Cancer. 2004;101:1947–57.
Rusby J, Brachtel E, Michaelson J, Koerner F, Smith B. Breast duct anatomy in the human nipple: three-dimensional patterns and clinical implications. Breast Cancer Res Treat. 2007;106:171–9.
Going JJ. Lobar anatomy of human breast and its importance for breast cancer. In: Tot T, editor. Breast cancer. London: Springer London; 2011. p. 19–38.
Eggeling H. Über ein wichtiges Stadium in der Entwicklung der menschlichen Milchdrüse. Anat Anz. 1904;24:595–605.
Renfree MB, Papenfuss AT, Deakin JE, Lindsay J, Heider T, Belov K, et al. Genome sequence of an Australian kangaroo, Macropus eugenii, provides insight into the evolution of mammalian reproduction and development. Genome Biol. 2011;12:R81. doi:10.1186/gb-2011-12-8-r81.
Veltmaat JM, Van Veelen W, Thiery JP, Bellusci S. Identification of the mammary line in mouse by Wnt10b expression. Dev Dyn. 2004;229:349–56.
Rein G. Untersuchungen über die embryonale Entwicklungsgeschichte der Milchdrüse I. Arch Mikrosk Anat. 1882;20:431–501. plates 28–29.
Gosling L. The duration of lactation in feral coypus (Myocastor coypus). J Zool. 1980;191:461–74.
Koyama S, Wu H-J, Easwaran T, Thopady S, Foley F. The nipple: a simple intersection of mammary gland and integument, but focal point of organ function. J Mammary Gland Biol Neoplasia. 2013;18. doi:10.1007/s10911-013-9289-1.
Pearl R. On the correlation between the number of mammá of the dam and size of litter in mammals. I. Interracial correlation. Exp Biol Med. 1913;11:27–30.
Gilbert AN. Mammary number and litter size in Rodentia: the “one-half rule”. Proc Natl Acad Sci U S A. 1986;83:4828–30.
Sherman PW, Braude S, Jarvis JUM. Littter sizes and mammary numbers of naked mole-rats: breaking the one-half rule. J Mammal. 1999;80:720–33.
Derocher AW. Supernumerary mammae and nipples in the polar bear (Ursus maritimus). J Mammal. 1990;71:236–7.
Hsu MJ, Moore J, Lin JF, Agoramoorthy G. High incidence of supernumerary nipples and twins in Formosan macaques (Macaca cyclopis) at Mt. Longevity, Taiwan. Am J Primatol. 2000;52:199–205.
Bell AG. Saving the six-nippled breed. Dr Bell’s last contribution to science. J Hered. 1923;14:99–111.
Castle WE. The genetics of multi-nippled sheep. An analysis of the sheep breeding experiments of Dr. and Mrs. Alexander Graham Bell at Beinn Bhreagh, N.S. J Hered. 1924;15:75–85.
Morrow GE, Nicol SC. Maternal care in the Tasmanian echidna (Tachyglossus aculeatus setosus ). Aust J Zool. 2013. doi:10.1071/ZO12066.
Pires-daSilva A, Sommer RJ. The evolution of signalling pathways in animal development. Nat Rev Genet. 2003;4:39–49.
Widelitz R, Chuong C-M. Early events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchyme. J Invest Dermatol Symp Proc. 1999;4:302–6.
Olivera-Martinez I, Viallet J, Michon F, Pearton DJ, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004;48:107–15.
McClellan HL, Miller SJ, Hartmann PE. Evolution of lactation: nutrition v. protection with special reference to five mammalian species. Nutr Res Rev. 2008;21:97–116.
Vorbach C, Capecchi MR, Penninger JM. Evolution of the mammary gland from the innate immune system? Bioessays. 2006;28:606–16.
Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–8.
Fliniaux I, Viallet J, Dhouailly D. Signaling dynamics of feather tract formation from the chick somatopleure. Development. 2004;131:3955–66.
Fliniaux I, Viallet J, Dhouailly D, Jahoda C. Transformation of amnion epithelium into skin and hair follicles. Int Soc Diff. 2004;72:558–65.
Prin F, Dhouailly D. How and when the regional competence of chick epidermis is established: feathers vs. scutate and reticulate scales, a problem en route to a solution. Int J Dev Biol. 2004;48:137–48.
Collomb E, Yang Y, Foriel S, Cadau S, Pearton DJ, Dhouailly D. The corneal epithelium and lens develop independently from a common pool of precursors. Dev Dyn. 2013. doi:10.1002/dvdy.23925.
Jussila M, Thesleff I. Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol. 2012; 4(4). doi:10.1101/cshperspect.a008425.
Veltmaat JM, Relaix F, Le LT, Kratochwil K, Sala FG, van Veelen W, et al. Gli3-mediated somitic FGF10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development. 2006;133:2325–35.
Huh SH, Närhi K, Lindfors PH, Häärä O, Yang L, Ornitz DM, et al. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Genes Dev. 2013;27(4):450–8.
Hens JR, Dann P, Zhang J, Harris S, Robinson G, Wysolmerski J. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development. 2007;134:1221–30.
Propper AY. Relations epidermo-mesodermiques dans la differenciation de l’ebauche mammaire d’embryon de lapin. Ann Embryol Morphogen. 1968;2:151–60.
Kratochwil K. Organ specificity in mesenchymal induction demonstrated in the embryonic development of the mammary gland of the mouse. Dev Biol. 1969;20:46–71.
Dhouailly D. [Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages]. J Embryol Exp Morphol. 1973;30:587–603.
Dhouailly D. Formation of cutaneous appendages in dermo-epidermal recombinations between reptiles, birds and mammals. Wilhelm Roux's Arch Dev Biol. 1975;177:323–40.
Dhouailly D. Dermo-epidermal interactions during morphogenesis of cutaneous appendages in amniotes. Front Matrix Biol. 1977;4:86–121.
Sick S, Reinker S, Timmer J, Schlake T. WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science. 2006;314(5804):1447–50.
Chu EY, Hens J, Andl T, Kairo A, Yamaguchi TP, Brisken C, et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development. 2004;131:4819–29.
Mikkola ML, Thesleff I. Ectodysplasin signaling in development. Cytokine Growth Factor Rev. 2003;14:211–24.
Harris M, Rohner N, Schwartz H, Perathoner H, Konstantinidis P, Nusslein-Volhard C. Zebrafish eda and edar mutants reveal conserved and ancestral roles of Ectodysplasin signaling in vertebrates. PLoS Genet. 2008;4. doi:10.1371/journal.pgen.1000206.
Huh S, Närhi K, Lindfors P, Häärä O, Yang L, Ornitz DM, et al. Fgf20 governs formation of primary and secondary dermal condensations in developing hair follicles. Gene Dev. 2013;27:450–8.
St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–68.
Pispa J, Pummila M, Barker PA, Thesleff I, Mikkola ML. Edar and Troy signalling pathways act redundantly to regulate initiation of hair follicle development. Hum Mol Genet. 2008;17:3380–91.
Lewis MT, Veltmaat JM. Next stop, the twilight zone: hedgehog network regulation of mammary gland development. J Mammary Gland Biol Neoplasia. 2004;9:165–81.
Gallego M, Beachy P, Hennighausen L, Robinson G. Differential requirements for Shh in mammary tissue and hair follicle morphogenesis. Dev Biol. 2002;249:131–9.
Michno K, Boras-Granic K, Mill P, Hui CC, Hamel PA. Shh expression is required for embryonic hair follicle but not mammary gland development. Dev Biol. 2003;264:153–65.
Hatsell SJ, Cowin P. Gli3-mediated repression of Hedgehog targets is required for normal mammary development. Development. 2006;133:3661–70.
Gritli-Linde A, Hallberg K, Harfe BD, Reyahi A, Kannius-Janson M, Nilsson J, et al. Abnormal hair development and apparent follicular transformation to mammary gland in the absence of hedgehog signaling. Dev Cell. 2007;12(1):99–112.
Chiang C, Swan R, Grachtchouk M, Bolinger M, Litingtung Y, Robertson E, et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev Biol. 1999;205:1–9.
Niemann C, Unden A, Lyle S, Zaouboulis C, Toftgård R, Watt F. Indian hedgehog and β-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003;30(Supplement 1):11873–80.
Hardy M. Glandular metaplasia of hair follicles and other responses to vitamin A excess in cultures of rodent skin. J Embryol Exp Morphol. 1968;19:157–80.
Viallet J, Dhouailly D. Retinoic acid and mouse skin morphogenesis. II. Role of epidermal competence in hair glandular metaplasia. Dev Biol. 1994;166:277–88.
Cho K, Kwon H, Shin J, Lee J, Cho S. Retinoic acid signaling and the initiation of mammary gland development. Dev Biol. 2012;365:259–66.
Parsa S, Ramasamy SK, De Langhe S, Gupte VV, Haigh JJ, Medina D, et al. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev Biol. 2008;317:121–31.
Unbekandt M, del Moral P, Sala FG, Bellusci S, Warburton D, Fleury V. Tracheal occlusion increases the rate of epithelial branching of embryonic mouse lung via the FGF10-FGFR2b-Sprouty2 pathway. Mech Dev. 2008;125:314–24.
Lo T, Yusoff P, Fong C, Guo K, McCaw B, Phillips W, et al. The ras/mitogen-activated protein kinase pathway inhibitor and likely tumor suppressor proteins, Sprouty 1 and Sprouty 2 are deregulated in breast cancer. Cancer Res. 2004;64:6127–36.
Kramer S, Okabe M, Hacohen N, Krasnow M, Hiromi Y. Sprouty: a common antagonist of FGF and EGF signaling pathways in Drosophila. Development. 1999;126:2515–25.
Yue Z, Jiang T, Wu P, Widelitz R, Chuong C-M. Sprouty/FGF signaling regulates the proximal-distal feather morphology and the size of dermal papillae. Dev Biol. 2012;372:45–54.
Foley J, Dann P, Hong J, Cosgrove J, Dreyer B, Rimm D, et al. Parathyroid hormone-related protein maintains mammary epithelial fate and triggers nipple skin differentiation during embryonic breast development. Development. 2001;128:513–25.
Wysolmerski JJ, Cormier S, Philbrick WM, Dann P, Zhang JP, Roume J, et al. Absence of functional type 1 parathyroid hormone (PTH)/PTH-related protein receptors in humans is associated with abnormal breast development and tooth impaction. J Clin Endocrinol Metab. 2001;86(4):1788–94.
Mayer JA, Foley J, De La Cruz D, Chuong C-M, Widelitz R. Conversion of the nipple to hair-bearing epithelia by lowering bone morphogenetic protein pathway activity at the dermal-epidermal interface. Am J Pathol. 2008;173:1339–48.
Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012;97:2947–56.
Debiais-Thibaud M, Oulion S, Bourrat F, Laurenti P, Casane D, Borday-Birraux V. The homology of odontodes in gnathostomes: insights from Dlx gene expression in the dogfish, Scyliorhinus canicula. BMC Evol Biol. 2011;11:307. doi:10.1186/1471-2148-11-307.
Kawasaki K, Lafont A, Sire J. The evolution of milk casein genes from tooth genes before the origin of mammals. Mol Biol Evol. 2011;28:2053–61.
Witzmann F, Scholz H, Mueller J, Kardjilov N. Sculpture and vascularization of dermal bones and implications for the physiology of basal tetrapods. Zool J Linnean Soc. 2010;160:302–40.
Wu P, Hou L, Plikus M, Hughes M, Scehnet J, Suksaweang S, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–70.
Alibardi L. Perspectives on hair evolution based on some comparative studies on vertebrate cornification. J Exp Zool Part B. 2012;318:325–43.
Lefèvre C, Sharp J, Nicholas K. Evolution of lactation: ancient origin and extreme adaptations of the lactation system. Annu Rev Genomics Hum Genet. 2010;11:219–38.
Widelitz RB, Veltmaat JM, Mayer JA, Foley J, Chuong CM. Mammary glands and feathers: comparing two skin appendages which help define novel classes during vertebrate evolution. Semin Cell Dev Biol. 2007;18(2):255–66.
Acknowledgments
We thank Dr. R. Eisert, F. Caraguel and Mrs. B. Peyrusse for assistance in figure preparation, and staff at the Smithsonian Institution Libraries and the History of Medicine Division of the US National Library of Medicine for locating obscure publications. We particularly acknowledge the Biodiversity Heritage Library for online access (www.biodiversitylibrary.org) to scans of old embryological publications. We are grateful to J. Veltmaat and B. Howard for many helpful comments on earlier versions of this paper.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental Table 1
(DOCX 31.1 kb)
Rights and permissions
About this article
Cite this article
Oftedal, O.T., Dhouailly, D. Evo-Devo of the Mammary Gland. J Mammary Gland Biol Neoplasia 18, 105–120 (2013). https://doi.org/10.1007/s10911-013-9290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10911-013-9290-8