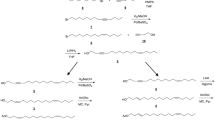
Abstract
Field experiments were carried out to study responses of male moths of the carpenterworm, Chilecomadia valdiviana (Lepidoptera: Cossidae), a pest of tree and fruit crops in Chile, to five compounds previously identified from the pheromone glands of females. Previously, attraction of males to the major component, (7Z,10Z)-7,10-hexadecadienal, was clearly demonstrated while the role of the minor components was uncertain due to the use of an experimental design that left large portions of the design space unexplored. We used mixture designs to study the potential contributions to trap catch of the four minor pheromone components produced by C. valdiviana. After systematically exploring the design space described by the five pheromone components, we concluded that the major pheromone component alone is responsible for attraction of male moths in this species. The need for appropriate experimental designs to address the problem of assessing responses to mixtures of semiochemicals in chemical ecology is described. We present an analysis of mixture designs and response surface modeling and an explanation of why this approach is superior to commonly used, but statistically inappropriate, designs.





Similar content being viewed by others
References
Allison JD, Cardé RT (2016) Pheromone communication in moths: evolution, behavior, and application. University of California Press, Oakland
Angulo AO, Olivares TS (1991) Chilecomadia valdiviana (Philippi) (Lepidoptera: Cossidae) asociado a Ulmus glabra Hudson forma pendula (Laud.) Rehder ("Olmo pendula") en la VIII Región (Concepción, Chile). Bosque 12:67–68
Angulo AO, Olivares TS (2008) Catálogo crítico e ilustrado de los Cossidae de Chile (Lepidoptera: Cossidae). Lepidoptera Novae 1:119–133
Artigas JN (1994) Entomología Económica. Insectos de interés agrícola, forestal, médico y veterinario, vol 2. Ediciones Universidad de Concepción, Chile, pp 479–486
Bestmann HJ, Stransky W, Vostrowsky O (1976) Darstellung lithiumsalzfreier Ylidlösungen mit Natrium-bis(trimethylsilyl)amid als base. Chem Ber 109:1694–1700
Cerda L, Angulo A, Durán A, Olivares T (2000) Insectos asociados a bosques del centro sur de Chile. In: Baldini A, Pancel L (eds) Agentes de daño en el bosque nativo. Editorial Universitaria, Santiago de Chile, pp 201–281
Cornell JA (2002) Experiments with mixtures, Third edn. Wiley, New York
Gentili P (1988) Análisis de la distribución geográfica de Cossidae (Lepidoptera: Ditrysia) de la Patagonia andina. Rev Chil Hist Nat 61:191–198
George J, Robbins PS, Alessandro RT, Stelinski LL, Lapointe SL (2016) Formic and acetic acids in degradation products of plant volatiles elicit olfactory and behavioral responses from an insect vector. Chem Senses 41:325–338
Herrera H, Barros-Parada W, Flores MF, Francke W, Fuentes-Contreras E, Rodriguez M, Santis F, Zarbin PHG, Bergmann J (2016) Identification of a novel moth sex pheromone component from Chilecomadia valdiviana (Lepidoptera: Cossidae). J Chem Ecol 42:908–918
Jordan TA, Zhang A, Pfeiffer DG (2013) Blend chemistry and field attraction of commercial sex pheromone lures to grape berry moth (Lepidoptera: Tortricidae), and a nontarget tortricid in vineyards. Environ Entomol 42:558–563
Kliejunas JT, Tkacz BM, Burdsall HH Jr, Denitto GA, Eglitis A, Haugen DA, Wallner WE (2001) Pest risk assessment of the importation into the United States of unprocessed Eucalyptus logs and chips from South America. Gen. TeC. Rep. FPL-GTR-124. Department of Agriculture, Forest Service, Forest Products Laboratory, Madison, WI: U.S., p 134
Knight AL, Barros-Parada W, Bosh D, Escudero-Colomar A, Fuentes-Contreras E, Hernández J, Kim Y, Kovanci OB, Levi A, Lo P, Molinari F, Valls J, Gemeno C (2015) Similar worldwide patterns in the sex pheromone signal and response in the oriental fruit moth, Grapholita molesta (Lepidoptera: Tortricidae). Bull Entomol Res 105:23–31
Knight AL, Basoalto E, Stelinski LL (2016) Variability in the efficacy of sex pheromone lures for monitoring oriental fruit moth (Lepidoptera: Tortricidae). J Appl Entomol 140:261–267
Lapointe SL, Evens TJ, Niedz RP (2008) Insect diets as mixtures: optimization for a polyphagous weevil. J Insect Physiol 54:1157–1167
Lapointe SL, Stelinksi LL, Evens TJ, Niedz RP, Hall DG, Mafra-Neto A (2009) Sensory imbalance as mechanism of mating disruption in the leafminer Phyllocnistis citrella: elucidation by multivariate geometric designs and response surface models. J Chem Ecol 35:896–903
Lapointe SL, Evens TJ, Niedz RP, Hall DG (2010a) Artificial diet optimized to produce normative adults of Diaprepes abbreviatus. Environ Entomol 39:670–677
Lapointe SL, Niedz RP, Evens TJ (2010b) An artificial diet for Diaprepes abbreviatus optimized for larval survival. Fla Entomol 93:56–62
Myers RH, Montgomery DC, Anderson-Cook CM (2016) Response surface methodology: process and product in optimization using designed experiments, 4th edn. John Wiley & Sons, Inc., New York
Niedz RP, Evens TJ (2016) Design of experiments (DOE) – history, concepts, and relevance to in vitro culture. In Vitro Cell Dev Biol Plant 52:547–562
Petersen JG (1988) Chilecomadia valdiviana (Philippi) (Lepidoptera: Cossidae), asociado a Nothofagus pumilio (Poepp. et Endl) Krasser (Lenga) en la Región de Magallanes. An Inst de la Patagonia 18:51–55
Piepel GF (1983) Defining consistent constraint regions in mixture experiments. Technometrics 25:97–101
Prado E (1991) Artrópodos y sus enemigos naturales asociados a plantas cultivadas en Chile. Boletín Técnico N° 169. Instituto de Investigaciones Agropecuarias, La Platina, p 203
Ripa R, Larral P (2008) Manejo de plagas en paltos y cítricos. Colección Libros INIA N° 23: La Cruz, Chile
Roelofs WL (1978) Threshold hypothesis for pheromone perception. J Chem Ecol 4:685–699
Scheffé H (1958) Experiments with Mixtures. J Roy Stat Soc Series B 20:344–360
Scheffé H (1963) Simplex-centroid designs for experiments with mixtures. J Roy Stat Soc Series B 25:235–263
Smith WF (2005) Experimental Design for Formulation. ASA-SIAM Series on Statistics and Applied Probability. SIAM, Philadelphia
Stuhl CJ, Cicero L, Sivinski J, Teal P, Lapointe S, Paranhos BJ, Aluja M (2011) Longevity of multiple species of tephritid (Diptera) fruit fly parasitoids (Hymenoptera: Braconidae: Opiinae) provided exotic and sympatric-fruit based diets. J Insect Physiol 57:1463–1470
Trimble RM, Marshall DB (2008) Relative attractiveness of incomplete and complete blends of synthetic pheromone to male obliquebanded leafroller (Lepidoptera: Tortricidae) moths in a flight tunnel and in apple orchards: implications for sex pheromone-mediated mating disruption of this species. Environ Entomol 37:366–373
Wang H-L, Svensson GP, Rosenberg O, Bengtsson M, Jirle EV, Löfstedt C (2010) Identification of the sex pheromone of the spruce seed moth, Cydia strobilella L. (Lepidoptera: Tortricidae). J. Chem. Ecol 36:305–313
Weisberg S (1985) Applied Linear Regression, 2nd edn. Wiley & Sons, New York
Witzgall P, Kirsch P, Cork A (2010) Sex pheromones and their impact on pest management. J Chem Ecol 36:80–100
Acknowledgements
Financial support from Fondo Nacional de Desarrollo Científico y Tecnológico (grant 1140779 to JB) is gratefully acknowledged. HH thanks Comisión Nacional de Investigación Científica y Tecnológica for a doctoral fellowship (21130375). USDA is an equal opportunity provider and employer. Mention of a trademark or proprietary product does not constitute a guarantee or warranty of the product by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may also be suitable.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 20 kb)
Rights and permissions
About this article
Cite this article
Lapointe, S.L., Barros-Parada, W., Fuentes-Contreras, E. et al. Use of Mixture Designs to Investigate Contribution of Minor Sex Pheromone Components to Trap Catch of the Carpenterworm Moth, Chilecomadia valdiviana . J Chem Ecol 43, 1046–1055 (2017). https://doi.org/10.1007/s10886-017-0906-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0906-0