Abstract
Inhalation sedation is increasingly performed in intensive care units. For this purpose, two anaesthetic reflectors, AnaConDa™ and Mirus™ are commercially available. However, their internal volume (100 ml) and possible carbon dioxide reflection raised concerns. Therefore, we compared carbon dioxide elimination of both with a heat moisture exchanger (HME, 35 ml) in a test lung model. A constant flow of carbon dioxide was insufflated into the test lung, ventilated with 500 ml, 10 breaths per minute. HME, Mirus and AnaConDa were connected successively. Inspired (insp-CO2) and end-tidal carbon dioxide concentrations (et-CO2) were measured under four conditions: ambient temperature pressure (ATP), body temperature pressure saturated (BTPS), BTPS with 0.4 Vol% (ISO-0.4), and 1.2 Vol% isoflurane (ISO-1.2). Tidal volume increase to maintain normocapnia was also determined. Insp-CO2 was higher with AnaConDa compared to Mirus and higher under ATP compared to BTPS. Isoflurane further decreased insp-CO2 and abolished the difference between AnaConDa and Mirus. Et-CO2 showed similar effects. In addition to volumetric dead space, reflective dead space was determined as 198 ± 6/58 ± 6/35 ± 0/25 ± 0 ml under ATP/BTPS/ISO-0.4/ISO-1.2 conditions for AnaConDa, and 92 ± 6/25 ± 0/25 ± 0/25 ± 0 ml under the same conditions for Mirus, respectively. Under BTPS conditions and with the use of moderate inhaled agent concentrations, reflective dead space is small and similar between the two devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Anaesthetic reflectors are increasingly used in intensive care units to sedate critically ill patients [1, 2]. Inhalation sedation has been implemented as an alternative sedation regimen in the Spanish, British, and German sedation guidelines [3,4,5]. Currently, two devices are commercially available, AnaConDa™ (Sedana Medical, Uppsala, Sweden), and Mirus™ (Pall Medical, Dreieich, Germany) [6, 7]. Chabanne et al. described an increase in the work of breathing as well as arterial carbon dioxide tension despite an increase in tidal volume when using AnaConDa during weaning off the ventilator [8]. It has been assumed that this may be caused by an increase in dead space as well as by partial carbon dioxide reflection as described by the group of Sturesson in a test lung model as well as in patients [9,10,11].
It was the aim of this study to quantify carbon dioxide elimination when using the AnaConDa and the Mirus system in comparison to a common heat moisture exchanger and to evaluate the influence of heat and moisture as well as the presence of isoflurane in a test lung model.
2 Methods
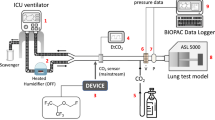
The experimental setup is shown in Fig. 1A. An Evita 4 ventilator (Dräger Medical, Lübeck, Germany), breathing hoses (Teleflex, Research Triangle Park, USA), the respective reflector (see below), a catheter mount with bronchoscopy port (Int’Air Medical, Bourg en Bresse, France), and a test lung (3 L manual breathing bag for Zeus®; Dräger Medical) were connected in line. Carbon dioxide was administered from a bottle with pressure reducer (Air Liquide Deutschland GmbH, Düsseldorf, Germany) via an oxygen application tube (Teleflex) and a flow meter (Rotameter®, Dräger Medical) through the bronchoscopy port into the test lung. Sample gas was drawn from the test lung side of the reflector into a gas monitor (Vamos®, Dräger Medical) connected with a personal computer for high resolution (every 10 ms) online storage of carbon dioxide and isoflurane concentrations. Sample gas from the monitor was redirected into the test lung. Breathing gas from the outlet of the ventilator was scavenged (FlurAbsorb®, Sedana Medical, Uppsala, Sweden). For experiments under body temperature pressure saturated conditions (BTPS), 50 ml distilled water were filled into the test lung which was placed into a heat insulated aquarium with 15 l distilled water heated up to 37 °C. Temperature and humidity of breathing gas were measured at the mouth of the test lung (Testo 610, Testo AG, Lenzkirch, Germany). For some experiments (see below), isoflurane (Forene®, AbbVie Deutschland, Ludwigshafen, Germany) was administered via a syringe pump with special syringe and tubing (Sedana Medical) through the bronchoscopy port directly into the test lung in order to compare the two reflectors in the same way. The bronchoscopy port containing three tubes was sealed with glue; leak tightness was checked with water.
A Experimental setup: the reflector to be evaluated (R) was connected between a ventilator and a test lung. Carbon dioxide (1) was administered from a bottle with pressure reducer through a flow meter into the test lung. Temperature and humidity were monitored at the mouth of the test lung (2). Gas was sampled from the patient side of the reflector (3) and then redirected into the test lung. A gas monitor stored gas concentrations online on a computer. The test lung was situated in a heat insulated aquarium partly filled with water. For some experiments, this water was heated up to 37 °C. For these experiments, some water was also filled into the test lung to achieve body temperature pressure saturated conditions. For some experiments, isoflurane (4) was pumped via a syringe pump into the test lung. B Experimental approach for measurement of inspiratory and end-tidal carbon dioxide concentrations (et-CO2) in Part I of the study. HME heat moisture exchanger, MIR Mirus™ reflector, ACD AnaConDa™ reflector. After adjusting carbon dioxide flow (CO2) into the test lung to keep et-CO2 at 5.3 ± 0.1 kPa for at least 5 min, as well as after 25 min equilibration after each exchange of a reflector, 5 min of high resolution recordings of carbon dioxide concentration equalling 50 breaths were stored on a personal computer
The Mirus system consists of a control unit connected via a multi-lumen cable with the Mirus exchanger, which is inserted between the Y-piece and the endotracheal tube. To avoid possible disturbing factors, the Mirus controller was not used and all lines between the control unit and the exchanger were interrupted and also sealed with glue.
Before each set of experiments, the gas monitor was calibrated and the ventilator was checked according to the manufacturer’s instructions. For all experiments, we used volume controlled ventilation with 500 ml tidal volume, 10 min−1 respiratory rate, 60 l min−1 constant flow, 1:1 inspiration to expiration ratio, 3 cm H2O positive end expiratory pressure, and 21% oxygen.
For each set of experiments, three types of ‘reflectors’ were tested in the following order:
-
1.
HME A normal heat moisture exchanger (HME, Humid-Vent® Filter Compact, straight, Teleflex) with 35 ml internal volume
-
2.
MIR Mirus exchanger with 100 ml internal volume
-
3.
ACD AnaConDa with 100 ml internal volume
We used four experimental conditions:
-
1.
ATP ambient pressure temperature: 20–23 °C, relative humidity in the test lung < 10%
-
2.
BTPS body temperature pressure saturated: 36.0–37.5 °C, relative humidity > 95%
-
3.
ISO-0.4 BTPS plus end-tidal isoflurane concentration 0.4 Vol%
-
4.
ISO-1.2 BTPS plus end-tidal isoflurane concentration 1.2 Vol%
2.1 Part I: carbon dioxide accumulation
With the HME in place, carbon dioxide flow was adjusted to result in 5.3 ± 0.1 kPa end-tidal carbon dioxide concentration (et-CO2) over at least 5 min with no further changes in flow (Fig. 1B). Then, gas concentrations from the HME were recorded during 5 min, equalling 50 breaths. Next, the HME was replaced by MIR. After 25 min of equilibration, gas concentrations were again recorded during 5 min. Thereafter, the HME was reconnected. After 25 min of equilibration, et-CO2 had to be 5.3 ± 0.1 kPa again, otherwise the experiment was repeated. Subsequently, ACD was tested the same way (see Fig. 1B).
HME, MIR, and ACD were all tested three times under each of the four conditions. For ISO-0.4 and ISO-1.2, isoflurane was started only when MIR or ACD were in place. The equilibration time was used to adjust the syringe pump rate to yield the respective end-tidal isoflurane concentration.
2.2 Part II: adjustment of tidal volume to keep et-CO2 at 5.3 ± 0.1 kPa
This time, when switching from HME to a reflector, tidal volume was adjusted stepwise to aim for et-CO2 of 5.3 ± 0.1 kPa (target). If after 10 breaths et-CO2 deviated from the target by more than 1 kPa (0.5 kPa/0.1 kPa), tidal volume was increased or decreased by 50 ml (20 ml/10 ml). The target had to be met during 5 min with no change in tidal volume. Tidal volume was recorded. Afterwards, the HME was reconnected; tidal volume of 500 ml was reinstalled. During 5 min, et-CO2 had to be 5.3 ± 0.1 kPa again, otherwise the experiment was repeated.
MIR and ACD were also tested in this part three times under each of the four conditions.
2.3 Data evaluation
In Part I, 36 high resolution recordings of carbon dioxide concentration, each comprising 50 breaths, were checked for artefacts. For each breath, minimal values were interpreted as inspiratory carbon dioxide concentration (insp-CO2) and maximum values as et-CO2. Data from the HME served as controls.
In Part II, 24 tidal volumes were determined: three for MIR and ACD under four conditions, respectively. For each, 500 ml tidal volume with the HME served as baseline.
To compensate for the higher internal volume of both reflectors (100 ml) compared to HME (35 ml), an increase by 65 ml was expected. The additional tidal volume increase, which is caused by carbon dioxide reflection, was calculated and referred to as reflective dead space.
Data are expressed as mean ± standard deviation. Differences between reflectors and conditions were analysed by One-way ANOVA, followed by post-hoc analysis including the correction of α error according to Bonferroni to compensate for multiple comparisons. Statistical significance was accepted at an error probability of p ≤ 0.05. All data analyses were performed using SPSS Statistics 19™ (IBM, Ehningen, Germany).
3 Results
3.1 Part I: carbon dioxide accumulation
Representative recordings of the carbon dioxide concentration during two breaths, with HME, MIR, and ACD after equilibration under all four conditions are shown (Fig. 2). Insp-CO2 with HME is close to zero, but not with MIR and ACD, indicating carbon dioxide reflection. Generally, et-CO2 with ACD is higher than with MIR, and with MIR it is higher than with HME. This effect is more distinct under ATP and is smallest when isoflurane is used under BTPS.
Representative recordings of carbon dioxide concentration (kPa) during two breaths, with different reflectors (HME heat moisture exchanger, MIR Mirus™, ACD AnaConDa™) under different conditions. ATP ambient temperature pressure, BTPS body temperature pressure saturated, ISO-0.4 BTPS plus isoflurane 0.4 Vol%, ISO-1.2 BTPS plus isoflurane 1.2 Vol%. a Full scale diagram, b enlarged scale of Y axis to show inspiratory concentrations
Insp-CO2 is significantly higher with both reflectors compared to HME (Fig. 3). It is significantly higher with ACD compared to MIR under ATP and BTPS, but not when isoflurane is used. Insp-CO2 is significantly lower with MIR and ACD during isoflurane administration compared to ATP and BTPS without isoflurane.
Inspiratory carbon dioxide concentration. Scatter dot plots with 150 measurements each (three series, 50 breaths each), mean with range, with different reflectors (HME heat moisture exchanger, MIR Mirus™, ACD AnaConDa™) under different conditions. ATP ambient temperature pressure, BTPS body temperature pressure saturated, ISO-0.4 BTPS plus isoflurane 0.4 Vol%, ISO-1.2 BTPS plus isoflurane 1.2 Vol%. *p < 0.05 versus HME. #p < 0.05 versus MIR. +p < 0.05 versus ATP. ‘p < 0.05 versus BTPS
Et-CO2 is significantly higher with both reflectors compared to HME (Fig. 4). It is significantly higher with ACD compared to MIR under ATP and BTPS. There is a small, but statistically significant difference between the two at the low, but not at the high isoflurane concentration. Et-CO2 is significantly lower with MIR and ACD during isoflurane administration compared to ATP and BTPS without isoflurane.
End-tidal carbon dioxide concentration. Scatter dot plots with 150 measurements each (three series, 50 breaths each), mean with range, with different reflectors (HME heat moisture exchanger, MIR Mirus™, ACD AnaConDa) under different conditions. ATP ambient temperature pressure, BTPS body temperature pressure saturated, ISO-0.4 BTPS plus isoflurane 0.4 Vol%, ISO-1.2 BTPS plus isoflurane 1.2 Vol%. *p < 0.05 versus HME. #p < 0.05 versus MIR. +p < 0.05 versus ATP. ‘p < 0.05 versus BTPS
3.2 Part II: adjustment of tidal volume to keep et-CO2 at 5.3 ± 0.1 kPa
To compensate for the higher internal volume of both reflectors (100 ml) compared to HME (35 ml), an increase by 65 ml was expected. The additional tidal volume increase, which is caused by carbon dioxide reflection, was 198 ± 6, 58 ± 6, 35 ± 0, and 25 ± 0 ml under ATP, BTPS, ISO-0.4, and ISO-1.2 conditions for ACD, as well as 92 ± 6, 25 ± 0, 25 ± 0, and 25 ± 0 ml under the same conditions for MIR, respectively (Table 1).
4 Discussion
This study shows that carbon dioxide elimination is hindered by both reflectors compared to a common HME. This effect cannot be totally explained by the internal volume of the reflectors (100 ml) which is 65 ml higher than that of the HME (35 ml) we used.
The significantly increased inspiratory carbon dioxide concentration of 1.2 kPa with AnaConDa and 0.4 kPa with Mirus shows that there is considerable carbon dioxide reflection by both reflectors under ATP conditions as are often used in simple lung models [9]. Interestingly, carbon dioxide reflection is considerably reduced under BTPS conditions, and it is again reduced when isoflurane is added to the breathing gas to about 0.2 kPa with both reflectors.
In turn, carbon dioxide reflection leads to its accumulation in the test lung, and after equilibration, end-tidal carbon dioxide is increased to more than 10 kPa with AnaConDa and to almost 8 kPa with Mirus compared to normocapnia with the HME. Again, when changing from ATP to BTPS and when adding isoflurane, this effect is dramatically reduced.
Impaired carbon dioxide elimination may best be explained by dead space ventilation. The dead space effects of both reflection devices may in theory be separated into volumetric (explained by its internal volume) as well as reflective device dead space.
In Part II of the study, we found considerable differences in reflective dead space between AnaConDa and Mirus, as well as between the different conditions.
Sturesson et al. compared dead space of an HME (internal volume 50 ml), an inactive (internal volume 100 ml without charcoal reflector), as well as an active AnaConDa reflector measured with the single breath test for carbon dioxide in a test lung under ATP conditions without volatile anaesthetics [9]. They found reflective dead space of the active reflector to be 180 ml which is similar to the 198 ml in our study.
In a second publication, the same group determined reflective dead space of AnaConDa in six postoperative patients without using volatile anaesthetics to be 86 ml which compares to 58 ml under BTPS conditions in our study [10].
In a third publication, this group evaluated the influence of different concentrations of sevoflurane on reflective dead space of AnaConDa [11]. In 12 postoperative patients, with the addition of 0.4 Vol% (0.8 Vol%) sevoflurane, reflective dead space decreased from 85 to 46 ml (38 ml) which compares to 35 ml under BTPS + 0.4 Vol% isoflurane in our study. Also, in a test lung under BTPS conditions, adding 0.6 Vol% sevoflurane decreased reflective dead space by 40 ml, but reflective dead space did not further decrease when increasing to 1.0, 2.0, and 3.0 Vol% sevoflurane. This, too, is consistent with our study: when increasing isoflurane concentration from 0.4 to 1.2 Vol%, there was only a marginal decrease with AnaConDa and no further decrease with Mirus.
All in all, the results of Sturesson’s group are similar to ours using a different, somewhat more simple and more straightforward clinically relevant method to determine reflective dead space. We also examined Mirus, and could show that Mirus also reflects carbon dioxide mainly under ATP conditions, although to a lesser extent than AnaConDa. When changing from ATP conditions, which are not clinically relevant, to BTPS and when adding volatile anaesthetics, reflective dead space becomes small (25–35 ml) and the difference between the two devices disappears. We also show that isoflurane has a similar, mitigating influence on carbon dioxide reflection as sevoflurane, as evaluated by Sturesson’s group.
Anatomical dead space amounts to approximately 140 ml in a 70 kg patient (2 ml/kg body weight). The endotracheal tube leads to a reduction of dead space compared to the amount of dead space of the pharynx plus oral or nasal cavity. Thus, anatomical (approximately 80 ml) [12], volumetric (100 ml), plus reflective device dead space (30 ml) add up to 210 ml dead space, leaving 140 ml tidal volume reaching the alveoli when the minimal tidal volume of 350 ml as declared in the manufacturer’s instructions for AnaConDa is employed [13]. Hypercapnia may develop. In all cases, additional devices such as nebulizers or tube elongations with large internal volumes should be avoided if possible and end-tidal carbon dioxide concentration should be closely monitored in all patients when starting the method. In patients with increased intracranial pressure, hypercapnia must be avoided.
Our data suggest that the Mirus reflector, which also consists of activated carbon, has different properties compared to AnaConDa. Different fractions of carbon that may be separated chemically may play a role. Reflection of volatile anaesthetics by Mirus is less efficient than by AnaConDa [6, 14]. Therefore, it is not surprising that Mirus also reflects less carbon dioxide. We speculate that hollow cavities in the activated carbon, acting as a molecular sieve, may be partly filled with water or anaesthetic molecules and thus can no longer take up carbon dioxide.
4.1 Limitations of the study
As this was a bench study with a test lung, we cannot be sure that carbon dioxide reflection of Mirus and AnaConDa will be exactly the same in patients. We did however establish BTPS conditions and also examined the addition of volatile anaesthetics which should be similar to the condition in patients. Also, beside test lung data, Sturesson’s group also presented patient data leading to similar results.
According to the manufacturer’s specifications, maximum measurements errors of the carbon dioxide concentration measured by the gas monitor as well as flow measurement of the ventilator to derive the tidal volume are in the order of 5%. Under constant laboratory conditions, error should be less. Great care was taken to identify possible drifts by going back to baseline after each measurement in which case measurements were repeated.
Volume adjustments in Part II of the study show large differences between both reflectors as well as between the different conditions. These results were reproducible, sometimes without any statistical deviations making statistical comparison impossible.
4.2 Clinical implications
Volumetric and reflective device dead space could be avoided altogether by using circle systems with carbon dioxide absorbers. Recently the use of a combination of a circle system together with an anaesthetic reflector has been described in animals [15]. However, such a system encompasses a large compressible volume which may deteriorate ventilator capabilities, trigger latencies and maximal flow generation. Chabanne et al. found an increased work of breathing in patients when using AnaConDa compared to baseline with active humidification, i.e. without HME [8]. Sevoflurane used for light sedation (on average: 0.5 Vol%) normalised respiratory parameters again. A likely reason for the increased work of breathing is device dead space. The authors acknowledged volumetric dead space of 100 ml plus 180 ml dead space caused by carbon dioxide reflection as published by Sturesson [9]. In fact, according to our data, when used in patients, reflective dead space would only be in the order of 60 ml. As a reason for the normalisation of respiratory parameters by sevoflurane, a bronchodilatory effect of sevoflurane is discussed. In fact, the addition of sevoflurane will have decreased reflective dead space which certainly also contributed to the normalisation of respiratory parameters. The authors conclude that sedation with sevoflurane and AnaConDa is possible during the weaning process.
Still, for the future it would be desirable to decrease reflective dead space by selecting reflection materials with higher specificity for anaesthetics and also to decrease volumetric dead space from 100 to 50 ml which is in the order of most heat moisture exchangers. Published data suggest that the reflection efficiency and capacity of AnaConDa are higher than needed for ICU sedation [16]. Consequently, the device could be miniaturised without clinically significant decrease in function.
5 Conclusion
Under BTPS conditions and with the use of moderate inhaled agent concentrations, reflective dead space is small and similar between Mirus and AnaConDa.
References
Jerath A, Parotto M, Wasowicz M, Ferguson ND. Volatile Anesthetics. Is a new player emerging in critical care sedation? Am J Respir Crit Care Med. 2016;193(11):1202–12. https://doi.org/10.1164/rccm.201512-2435CP.
Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the Anesthetic Conserving Device. Crit Care Med. 2004;32(11):2241–6.
Baron R, Binder A, Biniek R, Braune S, Buerkle H, Dall P, Demirakca S, Eckardt R, Eggers V, Eichler I, Fietze I, Freys S, Frund A, Garten L, Gohrbandt B, Harth I, Hartl W, Heppner HJ, Horter J, Huth R, Janssens U, Jungk C, Kaeuper KM, Kessler P, Kleinschmidt S, Kochanek M, Kumpf M, Meiser A, Mueller A, Orth M, Putensen C, Roth B, Schaefer M, Schaefers R, Schellongowski P, Schindler M, Schmitt R, Scholz J, Schroeder S, Schwarzmann G, Spies C, Stingele R, Tonner P, Trieschmann U, Tryba M, Wappler F, Waydhas C, Weiss B, Weisshaar G. (2015) Evidence and consensus based guideline for the management of delirium, analgesia, and sedation in intensive care medicine. Revision 2015 (DAS-guideline 2015)—short version. Ger Med Sci. 13:Doc19. https://doi.org/10.3205/000223.
Grounds M, Snelson C, Whitehouse T, Willson J, Tulloch L, Linhartova L, Shah A, Pierson R, England K. (2014) Intensive care society review of best practice for Analgesia and sedation in the critical care. Sedation Committee of the Intensive Care Society United Kingdom. http://www.ics.ac.uk/ICS/guidelines-and-standards.aspx. Accessed 6 Sept 2016.
Celis-Rodriguez E, Birchenall C, de la Cal MA, Castorena Arellano G, Hernandez A, Ceraso D, Diaz Cortes JC, Duenas Castell C, Jimenez EJ, Meza JC, Munoz Martinez T, Sosa Garcia JO, Pacheco Tovar C, Palizas F, Pardo Oviedo JM, Pinilla DI, Raffan-Sanabria F, Raimondi N, Righy Shinotsuka C, Suarez M, Ugarte S, Rubiano S. Clinical practice guidelines for evidence-based management of sedoanalgesia in critically ill adult patients. Medicina intensiva/Sociedad Espanola de Medicina Intensiva y Unidades Coronarias. 2013;37(8):519–74. https://doi.org/10.1016/j.medin.2013.04.001.
Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane—the Mirus. Anaesthesia. 2014;69(11):1241–50. https://doi.org/10.1111/anae.12798.
Bomberg H, Groesdonk HV, Bellgardt M, Volk T, Meiser A. AnaConDa and Mirus for intensive care sedation, 24 h desflurane versus isoflurane in one patient. SpringerPlus. 2016;5:420. https://doi.org/10.1186/s40064-016-2065-0.
Chabanne R, Perbet S, Futier E, Ben Said NA, Jaber S, Bazin JE, Pereira B, Constantin JM. Impact of the anesthetic conserving device on respiratory parameters and work of breathing in critically ill patients under light sedation with sevoflurane. Anesthesiology. 2014;121(4):808–16. https://doi.org/10.1097/aln.0000000000000394.
Sturesson LW, Malmkvist G, Bodelsson M, Niklason L, Jonson B. Carbon dioxide rebreathing with the anaesthetic conserving device, AnaConDa(R). Br J Anaesth. 2012;109(2):279–83. https://doi.org/10.1093/bja/aes102.
Sturesson LW, Bodelsson M, Johansson A, Jonson B, Malmkvist G. Apparent dead space with the anesthetic conserving device, AnaConDa(R): a clinical and laboratory investigation. Anesth Analg. 2013;117(6):1319–24. https://doi.org/10.1213/ANE.0b013e3182a7778e.
Sturesson LW, Bodelsson M, Jonson B, Malmkvist G. Anaesthetic conserving device AnaConDa: dead space effect and significance for lung protective ventilation. Br J Anaesth. 2014;113(3):508–14. https://doi.org/10.1093/bja/aeu102.
Oczenski W. Atmen-Atemhilfen. 10th ed. Thieme Verlag; 2017. p. 75–77.
Sedana, Medical. EN INSTRUCTIONS FOR USE AnaConDa® (Anaesthetic Conserving Device)—Administration system for anaesthetic agents. http://www.sedanamedical.com/files/IFU_Anaconda26000_English_151101.pdf. Accessed 6 Sept 2016.
Bomberg H, Wessendorf M, Bellgardt M, Veddeler M, Wagenpfeil S, Volk T, Groesdonk HV, Meiser A. Evaluating the efficiency of desflurane reflection in two commercially available reflectors. J Clin Monit Comput. 2016. https://doi.org/10.1007/s10877-016-9902-0.
Mashari A, Fisher JA, Fedorko L, Wasowicz M, Meineri M. Technology III: in-line vaporizer with reflector. J Clin Monit Comput. 2017. https://doi.org/10.1007/s10877-017-0078-z.
Meiser A, Bellgardt M, Belda J, Rohm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anaesthetic conserving device–a bench study with isoflurane and sevoflurane. J Clin Monit Comput. 2009;23(1):11–9. https://doi.org/10.1007/s10877-008-9158-4.
Acknowledgements
We thank Karen Schneider for critical revision and correction of language.
Funding
The Mirus™ control unit and all necessary disposables were kindly provided by the manufacturer Pall Medical, Dreieich, Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they did not receive any funding for realisation of this study. AM has received honoraria from Sedana Medical (Uppsala Sweden) and honoraria for lectures as well as research funding from Pall Medical (Dreieich, Germany).
Informed consent
No human participants or animals were involved.
Rights and permissions
About this article
Cite this article
Bomberg, H., Veddeler, M., Volk, T. et al. Volumetric and reflective device dead space of anaesthetic reflectors under different conditions. J Clin Monit Comput 32, 1073–1080 (2018). https://doi.org/10.1007/s10877-018-0105-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0105-8