Abstract
With the AnaConDa™ and the MIRUS™ system, volatile anesthetics can be administered for inhalation sedation in intensive care units. Instead of a circle system, both devices use anesthetic reflectors to save on the anesthetic agent. We studied the efficiency of desflurane reflection with both devices using different tidal volumes (VT), respiratory rates (RR), and ‘patient’ concentrations (CPat) in a bench study. A test lung was ventilated with four settings (volume control, RR × VT: 10 × 300 mL, 10 × 500 mL, 20 × 500 mL, 10 × 1000 mL). Two different methods for determination of reflection efficiency were established: First (steady state), a bypass flow carried desflurane into the test lung (flowin), the input concentration (Cin) was varied (1–17 vol%), and the same flow (flowex, Cex) was suctioned from the test lung. After equilibration, CPat was stored online and averaged; efficiency [%] was calculated \((100 \times \left( {1 - \left( {{\text{flow}}_{\text{in}} \times \left( {{\text{C}}_{\text{in}} - {\text{C}}_{\text{ex}} } \right)/{\text{C}}_{\text{Pat}} \times {\text{RR}} \times {\text{V}}_{\text{T}} } \right)} \right)\). Second (washout), flowin and flowex were stopped, the decline of CPat was measured; efficiency was calculated from the decay constant of the exponential regression equation. Both measurement methods yielded similar results (Bland–Altman: bias: −0.9 %, accuracy: ±5.55 %). Efficiencies higher than 80 % (>80 % of molecules exhaled are reflected) could be demonstrated in the clinical range of CPat and VT. Efficiency inversely correlates with the product of CPat and VT which can be imagined as the volume of anesthetic vapor exhaled by the patient in one breath, but not with the respiratory frequency. Efficiency of the AnaConDa™ was higher for each setting compared with the MIRUS™. Desflurane is reflected by both reflectors with efficiencies high enough for clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Inhalational sedation with the AnaConDa™ (Sedana Medical, Uppsala, Sweden) is frequently used for selected cases in some intensive care units [1, 2]. Instead of a circle system, the AnaConDa™ uses an anesthetic reflector between the Y-piece and the patient to save anesthetics. A gas monitor and a syringe pump delivering liquid isoflurane or sevoflurane are needed [3].
The new MIRUS™ system (TIM GmbH, Andernach, Germany, distributed by Pall Medical, Dreieich, Germany) also uses an anesthetic reflector. Its control unit measures gas concentrations and respiratory parameters. Anesthetics are delivered by injection into the device only during early inspiration [4]. Unlike the AnaConDa™, the MIRUS™ system is licensed for the administration of desflurane and can be used for this purpose.
Desflurane with its favorable kinetics and less metabolism compared to sevoflurane has become the preferred inhalational anesthetic in many operating rooms [5, 6]. Because of its low boiling point, desflurane is more complicated to deliver than isoflurane or sevoflurane. In a previous bench study, we found that the MIRUS™ is a useful and reliable system for administration of desflurane with a common intensive care ventilator. However, consumption was high [4].
Consumption of any volatile anesthetic depends on the mechanism of delivery, on uptake by the patient, and on elimination by the patient. Unlike other drugs elimination of volatiles takes place neither by metabolism nor by excretion through bile or urine, but through the patient’s ventilation. While the classical circle system reduces these losses via the ventilation by rebreathing of all gases after absorbing carbon dioxide, these new systems reflect the anesthetic specifically within the device. This is similar to a heat moisture exchanger reflecting only water while discarding all other gases expired by the patient.
In this study we focused on the efficiency of desflurane reflection by the respective reflectors of the AnaConDa™ and the Mirus™ system. We deliberately disregarded uptake by the patient by using a test balloon known not to take up volatile anesthetic. Uptake can be calculated by several physiological models and is usually low, especially with inhalation sedation where low concentrations are applied for longer time periods [7].
In contrast to our previous study, we also disregarded the mechanism of delivery as a factor possibly influencing consumption, and established a standardized constant delivery from a separate system directly into the test lung [4]. This methodological approach was necessary to measure and compare the efficiency of the reflector without any influence of delivery and uptake. Efficiency of reflection was defined as the percentage of molecules reflected back to the patient from all the molecules exhaled by the patient. In order to yield robust results, we used two different methods for determination of reflection efficiency.
Therefore, we compared the efficiency of the AnaConDa™ reflector with that of the MIRUS™ reflector using two independent methods.
2 Methods
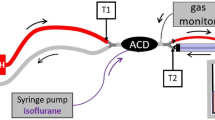
Setup and components of the AnaConDa™ and of the Mirus™ have been previously described [3, 4]. With the AnaConDa™, the infusion line for liquid anesthetic was left unused with its distal end blocked. Sample gas was redirected via the port normally used for gas sampling (Fig. 1a). With the Mirus™, the blue interface line was cut through, five inner lines were sealed with glue, and the one normally used for delivery of the anesthetic agent was used for redirecting the sample gas (Fig. 1b, c).
For all measurements an Evita 4 ventilator (Dräger Medical, Lübeck, Germany) was used with Volume Controlled Ventilation: 21 vol% inspired oxygen, 1:1 inspiration to expiration ratio, 60 L min−1 inspiratory flow with 0.05 s flow acceleration, and 3 mbar positive end-expiratory pressure. Four combinations of respiratory rate and tidal volume were tested: 10 × 300 mL, 10 × 500 mL, 20 × 500 mL, and 10 × 1000 mL. Prior to each series of measurements a calibration test was performed.
In order to yield robust results, two kinds of measurements were performed (Fig. 1d, e), one with stable concentrations after equilibration (“steady state”) and one during desflurane washout (“washout”).
As a test lung, we used a 3 L anesthesia bag made of Chloroprene (accessory for the Zeus Anesthesia Platform, Dräger Medical). Chloroprene is impermeable to and does not take up volatile anesthetics. The test lung was connected via an adapter (straight connector with T-Port, Medisize Deutschland GmbH, Neunkirchen-Seelscheid, Germany), a catheter mount (Int’Air Médical, Bourg-en-Bresse, France), the respective reflector (AnaConDa™ or MIRUS™), and the ventilation hoses (Anesthesia Breathing Circuits, Teleflex, Research Triangle Park, USA) to the ventilator. The gas outlet of the ventilator was connected to an active anesthesia gas scavenging system (Clean Air™, TIM GmbH, distributed by Pall Medical). Total volume of the test lung in expiration plus all pieces up to the Y-piece was 3148 mL. After setup, the whole system was tested for leaks.
To measure the concentration inside the test lung (termed ‘patient concentration’, Cpat), a gas sampling line (Dräger Medical) was inserted through the opening of the catheter mount with its tip in the middle of the test lung. Sample gas was redirected into the respective reflection device (see above). Data from the gas monitor (Vamos®, Dräger Medical) were stored online on a personal computer using the software Visia (Dräger Medical). Every 10 ms, the desflurane concentration was recorded.
2.1 Method 1
For the steady state measurements, we used the anesthesia system Primus™ with the desflurane vaporizer D-Vapor™ (both Dräger Medical) for washing in desflurane into the test lung. Connecting ports for the expiratory hose and the manual breathing bag of the Primus were blocked. The pressure limit was set to maximum pressure to avoid overflow. Thus, fresh gas, after having passed the vaporizer, was directed from the inspiratory port through an oxygen supply tubing (Teleflex) passing the catheter mount to the lower part of the test lung. Fresh gas flow was always set to 2 L min−1. The volume of desflurane vapor generated by the vaporizer per unit of time was added to yield the wash-in flow into the system (flowin, e.g. 2.410 L min−1 at 17 vol% desflurane).
Desflurane concentration in fresh gas (Cin) was measured with the gas analyzer integrated in the Primus anesthesia system. Sample gas is automatically redirected into the system. The dial of the vaporizer was adjusted to yield the pre-specified Cin in increasing order (2, 3, 4, 6, 8, 10, 12, and 17 vol%). With the MIRUS™ reflector, 1 vol% was also tested to see whether its desflurane reflection increases at very low concentrations.
To avoid a continuous, unidirectional flow through the reflector, gas was suctioned from the neck of the test lung through an oxygen supply tubing (Teleflex, flowex) via a mechanical flow-meter (Rotameter®, Dräger Medical) to an adjustable suctioning (Intermittent Suction Unit, Ohio Medical, Gurnee, IL, USA). Flowex was adjusted preliminarily with the flow-meter to equal flowin. Desflurane concentration in this gas was also measured by a Vamos gas monitor and stored online as specified above (Cex). The similarity of flowin and flowex was controlled by comparing the inspired and expired tidal volume displayed by the ventilator; for any deviation greater than 5 mL, the suctioning was readjusted.
To ensure steady state conditions, Cin, Cex, and Cpat had to be stable. The maximal deviation allowed for Cpat was a change of 5 % of its value over 10 min. Cpat and Cex were then averaged from the online recordings during steady state.
The net input of desflurane vapor into the test lung was calculated as follows:
As flowin equals flowex, this was simplified to:
Under steady state conditions, input of desflurane into the test lung must equal output or losses of desflurane through the reflector:
The efficiency of desflurane reflection was defined as:
with:
and:
Thus:
The product of tidal volume and patient concentration was termed (desflurane) vapor volume and plotted against reflection efficiency.
2.2 Method 2
For washout measurements, flowin and flowex were stopped by turning down fresh gas flow and suctioning, and by disconnecting and closing the respective lines (Fig. 1e). Ventilation of the test lung was continued unchanged and the decrease of Cpat was monitored with the gas monitor until it reached 10 % of the initial value. Data were stored as described above.
2.3 Data evaluation (washout measurements)
High resolution recordings of the decline of Cpat were plotted over time with the software Excel 2010 (Microsoft Corporation, Redmond, Washington, USA). For each interval from one whole-number concentration to the next (e.g. from 6 to 5 vol%), an exponential regression line was fitted to the data obtaining the corresponding time constant k R (decay constant with reflector). More calculations were performed in the lower concentration range because these intervals were passed more often.
From the exponential regression equation, k R was used for further calculations:
Assuming no reflection takes place, the Minute Volume (MV) generated by the ventilator will wash out desflurane from a total volume (V) of 3148 mL. The exponential washout curve will then be described by [8]:
(k NR : decay constant without reflector)
Efficiency of desflurane reflection can thus be calculated from the ratio of the decay constants:
2.4 Measurement errors
According to the manufacturer’s specifications, maximum measurement errors of gas concentration and flow measurements are in the order of 5 %. Moreover, steady state measurements with constant concentrations and flows, averaged over long time spans, will yield smaller errors than fluctuating measurements. With the washout method, calculation of the efficiency of reflection was reproducible and was based only on one gas concentration, measured over time, as well as on the total volume of the system which was a constant. In order to detect possible measurement errors, the results of both methods were compared with a Bland–Altman analysis.
2.5 Statistics
Continuous variables were expressed as mean and standard deviation (SD). The differences were compared using Student’s t tests (respectively Welch’s t tests in case of inhomogeneous variances). Statistical significance was accepted at p ≤ 0.05. Linearity between the efficiency of desflurane reflection and desflurane concentration was tested by Pearson correlation coefficients; differences between the groups were compared after Z transformation as described by Bortz [9]. Generalized estimating equations (GEE) were used to analyze the influence of tidal volume, respiratory rate, desflurane concentration, and AnaConDa™ and MIRUS™ reflector on the efficiency of desflurane reflection. Bland–Altman diagram was used to compare the efficiency of desflurane reflection of the steady state test and the washout test. Data were plotted in a Bland–Altman-diagram; accuracy and precision were calculated. All data analyses were performed using SPSS Statistics 19™ (IBM, Ehningen, Germany).
3 Results
3.1 Steady state
From the steady state measurements, 68 data points were determined: four ventilator settings times eight different input concentrations Cin with the AnaConDa™ and four ventilator settings times nine input concentrations Cin with the MIRUS™ (Fig. 2).
Steady state measurements. Efficiency of desflurane reflection depending on tidal volume for AnaConDa™ in comparison with MIRUS. Pearson: Pearson correlation coefficient. Differences of the Pearson’s correlation coefficients between the groups were compared after Z transformation: *p < 0.05 versus MIRUS™
The efficiency of desflurane reflection by the AnaConDa™ reflector decreased with an increase in desflurane concentration in the test lung (Pearson correlation coefficient: −0.955 to −0.998; p < 0.001; Fig. 2). Similarly, it decreased with an increase in tidal volume: The efficiency of 300 mL tidal volume was higher than 80 % over the whole concentration range up to 14 vol%. With 500 mL tidal volume and a frequency of 10 bpm, efficiency decreased to 80 % at 8.6 vol%. Interestingly, efficiency of 500 mL tidal volume and a frequency of 20 bpm were comparable to a frequency of ten. With 1000 mL tidal volume, efficiency decreased to 81 % at 3.3 vol%.
The efficiency of desflurane reflection by the MIRUS™ reflector also decreased with an increase in desflurane concentration in the test lung (Pearson correlation coefficient: −0.925 to −0.991; p < 0.001; Fig. 2). It also decreased with an increase in tidal volume. The results are comparable with the AnaConDa™ reflector. However, the AnaConDa™ reflector had higher efficiencies in each tidal volume compared with the MIRUS™ reflector.
The comparison of the Pearson correlation coefficient between AnaConDa™ and MIRUS™ was significantly different for 1000 mL (Pearson: −0.991 vs. −0.925; p = 0.04), but not for the smaller tidal volumes.
3.2 Washout
From the washout measurements, 164 data points were determined. The number of data points for each ventilator setting varied depending on the highest patient concentration CPat achieved.
The efficiency of desflurane reflection by the AnaConDa™ and MIRUS™ reflector was decreased with an increase in desflurane concentration and in tidal volume (GEE-test, p < 0.001) but not with an increase in frequency (Fig. 3). The AnaConDa™ reflector had a higher efficiency compared with the MIRUS™ reflector.
3.3 Vapor volume
We also found a significant inverse correlation between the efficiency of desflurane reflection and vapor volume (calculated as VT times CPat) for both the AnaConDa™ and the MIRUS™ reflector. Again, the AnaConDa™ reflector showed a greater efficiency of reflection compared with the MIRUS™ reflector (Fig. 4).
3.4 Accuracy and precision of concentration measurements
Efficiency of reflection measured by the steady state test conformed to that of the washout test with high accuracy (bias: −0.90 %) and precision (two SDs of measurement error: ±5.55 %, Fig. 5).
Bland Altman diagram for comparison of the efficiency of desflurane reflection measured by two types of measurements, one with stable concentrations after equilibration (“steady state”), one during desflurane washout (“washout”). The difference between the measurements for each efficiency of desflurane reflection is plotted against the mean of the two measurements. The bias (−0.90 %, dotted line) and random measurement disagreement (two standard deviations of measurement error: ±5.55 %, shaded area) are small
4 Discussion
In this bench study, we determined the efficiency of desflurane reflection by two commercially available reflection devices, AnaConDa™ and MIRUS™. We examined different tidal volumes, respiratory rates, and patient concentrations. In order to yield robust results, we used two different methods of measurements, “steady state” and “washout”. Both methods showed a good measurement agreement as evidenced by a Bland–Altman analysis.
We defined reflection efficiency as the percentage of molecules reflected back to the patient from all the molecules exhaled by the patient. If no reflection takes place, all molecules exhaled would be lost for the patient and consumption of anesthetic would be very high as in an open anesthesia system. An 80 % reflection means that consumption was 20 % of that of an open system. If reflection was close to 100 %, almost all molecules exhaled would be breathed in with the next inspiration. This would impair control of the concentration, when the concentration was to be decreased, unless the reflector would be removed or bypassed, which none of the devices is designed for. With this opposition of low consumption versus good control, efficiency of reflection is analogous to the fresh gas flow of conventional anesthesia systems, where a low fresh gas flow means low consumption but poor control. Therefore, from a clinical standpoint, efficiency should be somewhere between 80 and 100 %, at best around 90 % [4, 10].
We focused deliberately on anesthetic reflection and purposely excluded the influence of desflurane delivery. Pulsed injection of anesthetic during early inspiration as realized with the Mirus™ may by itself lower consumption of anesthetics as opposed to continuous delivery [4]. The geometry of the breathing circuit, especially the site of desflurane delivery, may also play a role.
In this study project, we showed a dependency of the desflurane reflection efficiency on the tidal volume and on the patient concentration. Interestingly, the respiratory frequency did not exert an influence. The product of tidal volume and concentration can be imagined as the volume of desflurane vapor exhaled by the patient with one breath. We found a strong almost linear inverse correlation between reflection efficiency and this calculated vapor volume. This strengthens the concept of the capacity of a reflector which we described previously for isoflurane and sevoflurane: When all binding sites on the reflector are occupied, additional anesthetic molecules will go through the reflector and will be lost to the patient, thus efficiency will be impaired [10].
This is also the first study to compare reflection efficiency of two commercially available reflectors. In this investigation, the AnaConDa™ showed higher efficiencies than the MIRUS™ reflector. More importantly, reflection efficiency of the AnaConDa™ was in the clinically acceptable range above 80 % over a wider range of tidal volumes and concentrations, i.e. up to 8.6 vol% with 500 mL tidal volume, up to 3.3 vol% with 1000 mL, whereas the MIRUS™ only worked well up to 2 vol% with 500 mL and 1 vol% with 1000 mL tidal volume (Fig. 3). This difference in efficiency between the two reflectors is probably not due to a different chemical composition, as both consist of activated carbon fibers according to the manufacturers. Different molecular shapes with differently sized cavities, the total amount of fibers and their three dimensional arrangement and thus the shape of the device, which could influence contact time, may all play a role.
With the AnaConDa™, reflection efficiency of desflurane was in the order of 90 % in the clinical range of tidal volumes and concentrations. In a previous bench study with the AnaConDa™ we found similar values for isoflurane and sevoflurane [10]. We did not examine reflection of isoflurane and sevoflurane with the Mirus™ as our focus was on desflurane reflection. Desflurane with its favorable kinetics and less metabolism compared to sevoflurane seems promising for inhalation sedation in the ICU [11]. Because of higher drug costs and higher concentrations needed, consumption of desflurane and therefore reflection efficiency is an important issue.
In order to evaluate the efficiency of a reflection system for desflurane, several modes of delivery seem possible: With a syringe cooled with ice, desflurane can be injected as a liquid with a syringe pump. We performed such a test series, but formation of gas bubbles led to inconsistent delivery and increased variability of the results [12]. Another possibility is to add a bypass flow with anesthetic vapor to the breathing system as described by Sturesson et al. [13].
These authors found reflection efficiencies of granulated charcoal and zeolite pellets to be around 70 % for 1,5 and 3.0 vol% desflurane and around 60 % for 6 vol% desflurane (with a tidal volume of 600 mL). However, with this method, an additional flow bypassing the reflector during inspiration was needed. This leads to a higher tidal volume expired through the reflector and an increased washout of desflurane during expiration which could reduce efficiency. To avoid the increased washout during expiration, we used two different methods. At first, we added a flow for the delivery of desflurane, but at the same time subtracted the same flow from the system to avoid an additional unidirectional flow through the reflector. Secondly, we simply washed out desflurane with no further delivery.
The lower efficiency of reflection described by Sturesson et al. [13] compared to our study may also be explained by the fact that we used commercial reflectors with optimized properties such as enlarged surface and optimal shape, whereas Sturesson et al. used handmade reflectors.
There is a limitation of our study that several measurements all with possible measurement errors were combined in the calculations. However, the first and the second method yielded reproducible and very similar results. The comparison of both methods in the Bland–Altman analysis showed high accuracy and precision.
Because this investigation was performed in a bench site model, the results may not be directly transferable to humans. To avoid further confounders, all experiments were performed under ambient temperature pressure conditions in absence of carbon dioxide.
5 Conclusion
This study showed that desflurane, despite its lower boiling point, is reflected by two commercially available reflectors with efficiencies high enough for clinical use. Efficiencies higher than 80 % (i.e. more than 80 % of molecules exhaled by the patient are reflected) could be demonstrated in the clinical range of patient concentrations and tidal volumes. Efficiency of reflection inversely correlates with the product of patient concentration and tidal volume which can be considered as the volume of anesthetic vapor exhaled by the patient in one breath. Efficiency of the AnaConDa™ is superior to that of the MIRUS™, however, desflurane can only be administered with the MIRUS™ system. Therefore, a Mirus™ with an improved reflector, or an AnaConDa™ with the possibility to administer desflurane, would be clinically desirable.
References
Bellgardt M, Bomberg H, Herzog-Niescery J, Dasch B, Vogelsang H, Weber TP, Steinfort C, Uhl W, Wagenpfeil S, Volk T, Meiser A. Survival after long-term isoflurane sedation as opposed to intravenous sedation in critically ill surgical patients. Eur J Anaesthesiol. 2015;33:6–13.
Sackey PV, Martling CR, Granath F, Radell PJ. Prolonged isoflurane sedation of intensive care unit patients with the anesthetic conserving device. Crit Care Med. 2004;32:2241–6.
Meiser A, Laubenthal H. Inhalational anaesthetics in the ICU: theory and practice of inhalational sedation in the ICU, economics, risk-benefit. Best Pract Res Clin Anaesthesiol. 2005;19:523–38.
Bomberg H, Glas M, Groesdonk VH, Bellgardt M, Schwarz J, Volk T, Meiser A. A novel device for target controlled administration and reflection of desflurane: the Mirus. Anaesthesia. 2014;69:1241–50.
Bennett JA, Lingaraju N, Horrow JC, McElrath T, Keykhah MM. Elderly patients recover more rapidly from desflurane than from isoflurane anesthesia. J Clin Anesth. 1992;4(5):378–81.
Rohm KD, Mengistu A, Boldt J, Mayer J, Beck G, Piper SN. Renal integrity in sevoflurane sedation in the intensive care unit with the anesthetic-conserving device: a comparison with intravenous propofol sedation. Anesth Analg. 2009;108:1848–54.
Belda JF, Soro M, Badenes R, Meiser A, Garcia ML, Aguilar G, Marti FJ. The predictive performance of a pharmacokinetic model for manually adjusted infusion of liquid sevofluorane for use with the Anesthetic-Conserving Device (AnaConDa): a clinical study. Anesth Analg. 2008;106:1207–14.
Cruickshank S. Mathematics and statistics in anaesthesia. New York: Oxford University Press; 1998.
Bortz J. Statistik für Sozialwissenschaftler. Berlin: Springer; 1993.
Meiser A, Bellgardt M, Belda J, Rohm K, Laubenthal H, Sirtl C. Technical performance and reflection capacity of the anaesthetic conserving device: a bench study with isoflurane and sevoflurane. J Clin Monit Comput. 2009;23:11–9.
Meiser A, Sirtl C, Bellgardt M, Lohmann S, Garthoff A, Kaiser J, Hugler P, Laubenthal HJ. Desflurane compared with propofol for postoperative sedation in the intensive care unit. Br J Anaesth. 2003;90:273–80.
Hartmuth N (2014) Effizienz des “Anaesthetic conserving device” (AnaConDa(TM)): Ein Modellversuch mit Desfluran. Dissertation, Ruhr-Universität Bochum.
Sturesson LW, Frennstrom JO, Ilardi M, Reinstrup P. Comparing charcoal and zeolite reflection filters for volatile anaesthetics: a laboratory evaluation. Eur J Anaesthesiol. 2015;32:521–6.
Acknowledgments
We thank Karen Schneider for critical revision and correction of language
Funding
The Mirus™ control unit and all necessary disposables were kindly provided by the manufacturer Pall medical, Dreieich, Germany.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they did not receive any funding for realization of this study.
Rights and permissions
About this article
Cite this article
Bomberg, H., Wessendorf, M., Bellgardt, M. et al. Evaluating the efficiency of desflurane reflection in two commercially available reflectors. J Clin Monit Comput 32, 605–614 (2018). https://doi.org/10.1007/s10877-016-9902-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9902-0