Abstract
The tetraruthenium tetrahydrido carbonyl cluster Ru4(µ-H)4(CO)12 (1) reacts with diphenylvinylphosphine under thermal activation to give the substitution products Ru4(µ-H)4(CO)12−n(κ1-Ph2PCH=CH2)n, (2) (n = 3 (a); 4 (b)). Both 2a and 2b have been completely characterized, including by single-crystal X-ray diffraction analysis. Each of the diphenylvinylphosphine ligands in 2a and 2b is terminally bound, via the phosphorus atom, to a different ruthenium metal center, while the hydride positions in the Ru4(µ-H)4 cores retain the D2d symmetry of the parent cluster 1. Clusters 2a and 2b were able to retain their structural integrity at elevated temperatures.
Graphic Abstract
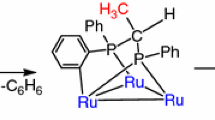
Thermolysis of Ru4(μ-H)4(CO)12 (1) in cyclohexane with diphenylvinylphosphine afforded the tri- and tetra-substituted derivatives Ru4(μ-H)4(CO)9(κ1-Ph2PCH=CH2)3 (2a) and Ru4(μ-H)4(CO)8(κ1-Ph2PCH=CH2)4 (2b). For both substituted clusters, the diphenylvinylphosphine ligands are bound terminally to the tetraruthenium core via the phosphorus atoms while the hydride positions retain the D2d symmetry of the parent cluster 1.




Similar content being viewed by others
References
A. Bader and E. Lindner (1991). Coord. Chem. Rev. 108, 27.
P. Espinet and K. Soulantica (1999). Coord. Chem. Rev. 193–195, 499.
P. Braunstein and F. Naud (2001). Angew. Chem. Int. Ed. 40, 680.
V. V. Grushin (2004). Chem. Rev. 104, 1629.
P. Braunstein (2004). J. Organomet. Chem. 689, 3953.
H. Grützmacher (2008). Angew. Chem. Int. Ed. 47, 1814.
X. R. L. Fontain, D. P. Markham, and B. L. Shaw (1990). J. Organomet. Chem. 391, 123.
G. Hogarth (1991). J. Organomet. Chem. 407, 91.
D. Peña, Y. Otero, A. Arce, L. Díaz, Y. De Sanctis, E. Ocando-Mavarez, J. M. Garcia, R. Machado, and T. González (2014). J. Organomet. Chem. 772–773, 7.
D. Peña, Y. Otero, A. Arce, J. M. Garcia, D. S. Coll, E. Ocando-Mavarez, R. Machado, and T. González (2016). Inorg. Chim. Acta 439, 178.
E. V. Grachova, M. Haukka, B. T. Heaton, E. Nordlander, T. A. Pakkanen, I. S. Podkorytov, and S. P. Tunik (2003). Dalton Trans. 12, 2468.
R. Gobetto, E. Sappa, A. Tiripicchio, M. T. Camellini, and M. J. Mays (1990). J. Chem. Soc. Dalton Trans., 807.
B. F. G. Johnson, J. Lewis, E. Nordlander, and P. R. Raithby (1996). J. Chem. Soc. Dalton Trans. 19, 3825.
R. Giordano, E. Sappa, G. Predieri, and A. Tiripicchio (1997). J. Organomet. Chem. 547, 49.
M. Bianchi, G. Menchi, P. Frediani, U. Matteoli, and F. Piacenti (1983). J. Organomet. Chem. 247, 89.
U. Matteoli, M. Bianchi, P. Frediani, F. Piacenti, C. Botteghi, and M. Marchetti (1984). J. Organomet. Chem. 263, 243.
C. Botteghi, S. Gladiali, M. Bianchi, U. Matteoli, P. Frediani, P. G. Vergamini, and E. Benedetti (1977). J. Organomet. Chem. 140, 221.
M. Bianchi, U. Matteoli, G. Menchi, F. Gloria, P. Frediani, F. Piacenti, and C. Botteghi (1980). J. Organomet. Chem. 195, 337.
U. Matteoli, G. Menchi, P. Frediani, M. Bianchi, and F. Piacenti (1985). J. Organomet. Chem. 28, 281.
U. Matteoli, V. Beghetto, and A. Scrivanti (1996). J. Mol. Catal. A 109, 45.
R. D. Adams, E. M. Boswell, B. Captain, A. B. Hungria, P. A. Midgley, R. Raja, and J. M. Thomas (2007). Angew. Chem. Int. Ed. 46, 8182.
L. Howard-Fabretto and G. G. Andersson (2019). Adv. Mater. 32, 1904122.
R. D. Adams, E. M. Boswell, B. Captain, and M. A. Patel (2007). Inorg. Chem. 46, 533.
G. Hogarth, S. E. Kabir, and E. Nordlander (2010). Dalton Trans. 39, 6153.
P. Homanen, R. Persson, M. Haukka, T. A. Pakkanen, and E. Nordlander (2000). Organometallics 19, 5568.
V. Moberg, P. Homanen, S. Selva, R. Persson, M. Haukka, T. A. Pakkanen, M. Monari, and E. Nordlander (2006). Dalton Trans. 1, 279.
V. Moberg, M. Haukka, I. O. Koshevoy, R. Ortiz, and E. Nordlander (2006). Organometallics 26, 4090.
V. Moberg, R. Duquesne, S. Contaldi, O. Röhrs, J. Nachtigall, L. Damoense, A. T. Hutton, M. Green, M. Monari, D. Santelia, M. Haukka, and E. Nordlander (2012). Chem. Eur. J. 18, 12458.
M. G. Ballinas-López, E. V. García-Báez, and M. J. Rosales-Hoz (2003). Polyhedron 22, 3403.
R. K. Pomeroy (1995). in: G. Wilkinson, F. G. A. Stone, E. W. Abel (eds.), Comprehensive Organometallic Chemistry II (Pergamon, Oxford, New York, 1995), vol. 7, ch. 15.
M. R. Churchill and B. G. DeBoer (1977). Inorg. Chem. 16, 878.
B. F. G. Johnson, J. Lewis, P. R. Raithby, G. M. Sheldrick, K. Wong, and M. McPartlin (1978). J. Chem. Soc. Dalton Trans., 673.
M. B. Smith and J. March, March’s Advanced Organic Chemistry, 5th ed. (Wiley, New York, 2001), p. 20.
S. Aime, M. Botta, R. Gobetto, L. Milone, D. Osella, R. Gellert, and E. Rosenberg (1995). Organometallics 14, 3693.
S. A. R. Knox and H. D. Kaesz (1971). J. Am. Chem. Soc. 93, 4594.
S. Aime, M. Botta, R. Gobetto, and D. Osella (1986). Inorg. Chim. Acta 115, 129.
A. G. Orpen and R. K. McMullan (1983). J. Chem. Soc. Dalton Trans., 463.
M. G. Ballinas-López, M. J. Rosales-Hoz, and E. V. García-Báez (2003). Inorg. Chem. Commun. 6, 675.
M. Bianchi, P. Frediani, A. Salvani, L. Rosi, L. Pistolesi, F. Piacenti, S. Ianelli, and M. Nardelli (1997). Organometallics 16, 482.
M. I. Bruce, B. K. Nicholson, J. M. Patrick, and A. H. White (1983). J. Organomet. Chem. 254, 361.
S. A. R. Knox, J. W. Koepke, M. A. Andrews, and H. D. Kaesz (1975). J. Am. Chem. Soc. 97, 3942.
SMART, version 5.628; Bruker AXS, Inc., Madison, WI, USA, 2001.
SAINT+, version 6.22a; Bruker AXS, Inc., Madison, WI, USA, 2001.
G. M. Sheldrick (1996). SADABS.
G. M. Sheldrick (2015). Acta Cryst. C 71, 3.
A. G. Orpen (1980). J. Chem. Soc. Dalton Trans., 2509.
Acknowledgments
This work was supported by Hwa Chong Institution as well as Nanyang Technological University and the Ministry of Education with a research Grant (No. M4011158).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10876_2021_2150_MOESM1_ESM.pdf
Supplementary file1 (PDF 518 kb) Supplementary Material: CCDC 1517034‒1517035 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif. IR, NMR and ESI–MS spectra for 2a and 2b.
Rights and permissions
About this article
Cite this article
Neo, R.K.E., Ong, H.W., Wong, Z.X. et al. Coordination of the Hemilabile Ligand Diphenylvinylphosphine to Ru4(µ-H)4(CO)12: Synthesis, Stability and Structural Studies. J Clust Sci 33, 2337–2343 (2022). https://doi.org/10.1007/s10876-021-02150-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-021-02150-0