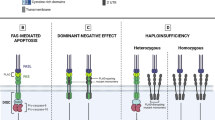
Abstract
The autoimmune lymphoproliferative syndrome (ALPS) is a non-malignant and non-infectious uncontrolled proliferation of lymphocytes accompanied by autoimmune cytopenia. The genetic etiology of the ALPS was described in 1995 by the discovery of the FAS gene mutations. The related apoptosis defect accounts for the accumulation of autoreactive lymphocytes as well as for specific clinical and biological features that distinguish the ALPS-FAS from other monogenic defects of this apoptosis pathway, such as FADD and CASPASE 8 deficiencies. The ALPS-FAS was the first description of a monogenic cause of autoimmunity, but its non-Mendelian expression remained elusive until the description of somatic and germline mutations in ALPS patients. The recognition of these genetic diseases brought new information on the role of this apoptotic pathway in controlling the adaptive immune response in humans.


Similar content being viewed by others
References
Canale VC, Smith CH. Chronic lymphadenopathy simulating malignant lymphoma. J Pediatr. 1967;70(6):891–9.
Lavrik IN, Krammer PH. Regulation of CD95/Fas signaling at the DISC. Cell Death Differ. 2012;19(1):36–41. https://doi.org/10.1038/cdd.2011.155.
Schleich K, Krammer PH, Lavrik IN. The chains of death: a new view on caspase-8 activation at the DISC. Cell Cycle. 2013;12(2):193–4. https://doi.org/10.4161/cc.23464.
Rieux-Laucat F, Fischer A, Deist FL. Cell-death signaling and human disease. Curr Opin Immunol. 2003;15(3):325–31.
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defect in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7.
Yonehara S, Ishii A, Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989;169(5):1747–56.
Trauth B, Klas C, Peters A, Matzku S, Möller P, Falk W, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245:301–4.
Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148(5):1198–215.
Takei F. Unique surface phenotype of T cells in lymphoproliferative autoimmune MRL/Mp-lpr/lpr mice. J Immunol. 1984;133(4):1951–4.
Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol Today. 1995;16(1):39–43.
Shi X, Xie C, Kreska D, Richardson JA, Mohan C. Genetic dissection of SLE: SLE1 and FAS impact alternate pathways leading to lymphoproliferative autoimmunity. J Exp Med. 2002;196(3):281–92.
Vidal S, Kono DH, Theofilopoulos AN. Loci predisposing to autoimmunity in MRL-Fas lpr and C57BL/6-Faslpr mice. J Clin Invest. 1998;101(3):696–702.
Adachi M, Suematsu S, Suda T, Watanabe D, Fukuyama H, Ogasawara J, et al. Enhanced and accelerated lymphoproliferation in Fas-null mice. Proc Natl Acad Sci U S A. 1996;93(5):2131–6.
Karray S, Kress C, Cuvellier S, Hue-Beauvais C, Damotte D, Babinet C, et al. Complete loss of Fas ligand gene causes massive lymphoproliferation and early death, indicating a residual activity of gld allele. J Immunol. 2004;172(4):2118–25.
Matsuzawa A, Moriyama T, Kaneko T, Tanaka M, Kimura M, Ikeda H, et al. A new allele of the lpr locus, lprcg, that complements the gld gene in induction of lymphadenopathy in the mouse. J Exp Med. 1990;171(2):519–31.
Fisher GH, Rosenberg FJ, Straus SE, Dale JK, Middleton LA, Lin AY, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81(6):935–46.
Rieux-Laucat F, Le Deist F, Hivroz C, Roberts I, Debatin K, Fischer A, et al. Mutations in fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9.
Le Deist F, Emile JF, Rieux-Laucat F, Benkerrou M, Roberts I, Brousse N, et al. Clinical, immunological, and pathological consequences of Fas-deficient conditions. Lancet. 1996;348(9029):719–23. https://doi.org/10.1016/S0140-6736(96)02293-3.
Bettinardi A, Brugnoni D, Quiros-Roldan E, Malagoli A, La Grutta S, Correra A, et al. Missense mutations in the Fas gene resulting in autoimmune lymphoproliferative syndrome: a molecular and immunological analysis. Blood. 1997;89(3):902–9.
Holzelova E, Vonarbourg C, Stolzenberg MC, Arkwright PD, Selz F, Prieur AM, et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351(14):1409–18. https://doi.org/10.1056/NEJMoa040036.
Dowdell KC, Niemela JE, Price S, Davis J, Hornung RL, Oliveira JB, et al. Somatic FAS mutations are common in patients with genetically undefined autoimmune lymphoproliferative syndrome. Blood. 2010;115(25):5164–9. https://doi.org/10.1182/blood-2010-01-263145.
Savic S, Dickie LJ, Battellino M, McDermott MF. Familial Mediterranean fever and related periodic fever syndromes/autoinflammatory diseases. Curr Opin Rheumatol. 2012;24(1):103–12. https://doi.org/10.1097/BOR.0b013e32834dd2d5.
Ma CA, Xi L, Cauff B, DeZure A, Freeman AF, Hambleton S, et al. Somatic STAT5b gain-of-function mutations in early onset nonclonal eosinophilia, urticaria, dermatitis, and diarrhea. Blood. 2017;129(5):650–3. https://doi.org/10.1182/blood-2016-09-737817.
Magerus-Chatinet A, Neven B, Stolzenberg MC, Daussy C, Arkwright PD, Lanzarotti N, et al. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Invest. 2011;121(1):106–12. https://doi.org/10.1172/JCI43752.
Martinez-Feito A, Melero J, Mora-Diaz S, Rodriguez-Vigil C, Elduayen R, Gonzalez-Granado LI, et al. Autoimmune lymphoproliferative syndrome due to somatic FAS mutation (ALPS-sFAS) combined with a germline caspase-10 (CASP10) variation. Immunobiology. 2016;221(1):40–7. https://doi.org/10.1016/j.imbio.2015.08.004.
van der Burg M, de Groot R, Comans-Bitter WM, den Hollander JC, Hooijkaas H, Neijens HJ, et al. Autoimmune lymphoproliferative syndrome (ALPS) in a child from consanguineous parents: a dominant or recessive disease? Pediatr Res. 2000;47(3):336–43.
Kasahara Y, Wada T, Niida Y, Yachie A, Seki H, Ishida Y, et al. Novel Fas (CD95/APO-1) mutations in infants with a lymphoproliferative disorder. Int Immunol. 1998;10(2):195–202.
Del-Rey M, Ruiz-Contreras J, Bosque A, Calleja S, Gomez-Rial J, Roldan E, et al. A homozygous Fas ligand gene mutation in a patient causes a new type of autoimmune lymphoproliferative syndrome. Blood. 2006;108(4):1306–12. https://doi.org/10.1182/blood-2006-04-015776.
Magerus-Chatinet A, Stolzenberg MC, Lanzarotti N, Neven B, Daussy C, Picard C, et al. Autoimmune lymphoproliferative syndrome caused by a homozygous null FAS ligand (FASLG) mutation. J Allergy Clin Immunol. 2013;131(2):486–90. https://doi.org/10.1016/j.jaci.2012.06.011.
Sobh A, Crestani E, Cangemi B, Kane J, Chou J, Pai SY, et al. Autoimmune lymphoproliferative syndrome caused by a homozygous FasL mutation that disrupts FasL assembly. J Allergy Clin Immunol. 2015;137:324–327.e2. https://doi.org/10.1016/j.jaci.2015.08.025.
Nabhani S, Honscheid A, Oommen PT, Fleckenstein B, Schaper J, Kuhlen M, et al. A novel homozygous Fas ligand mutation leads to early protein truncation, abrogation of death receptor and reverse signaling and a severe form of the autoimmune lymphoproliferative syndrome. Clin Immunol. 2014;155(2):231–7. https://doi.org/10.1016/j.clim.2014.10.006.
Suzuki I, Fink PJ. Maximal proliferation of cytotoxic T lymphocytes requires reverse signaling through Fas ligand. J Exp Med. 1998;187(1):123–8.
Luckerath K, Kirkin V, Melzer IM, Thalheimer FB, Siele D, Milani W, et al. Immune modulation by Fas ligand reverse signaling: lymphocyte proliferation is attenuated by the intracellular Fas ligand domain. Blood. 2011;117(2):519–29. https://doi.org/10.1182/blood-2010-07-292722.
Paulsen M, Mathew B, Qian J, Lettau M, Kabelitz D, Janssen O. FasL cross-linking inhibits activation of human peripheral T cells. Int Immunol. 2009;21(5):587–98. https://doi.org/10.1093/intimm/dxp028.
Desbarats J, Duke RC, Newell MK. Newly discovered role for Fas ligand in the cell-cycle arrest of CD4+ T cells. Nat Med. 1998;4(12):1377–82. https://doi.org/10.1038/3965.
Malleter M, Tauzin S, Bessede A, Castellano R, Goubard A, Godey F, et al. CD95L cell surface cleavage triggers a prometastatic signaling pathway in triple-negative breast cancer. Cancer Res. 2013;73(22):6711–21. https://doi.org/10.1158/0008-5472.CAN-13-1794.
Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, et al. Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell. 2008;13(3):235–48. https://doi.org/10.1016/j.ccr.2008.02.003.
Poissonnier A, Sanseau D, Le Gallo M, Malleter M, Levoin N, Viel R, et al. CD95-mediated calcium signaling promotes T helper 17 trafficking to inflamed organs in lupus-prone mice. Immunity. 2016;45(1):209–23. https://doi.org/10.1016/j.immuni.2016.06.028.
Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270(5239):1189–92.
Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377(6550):630–2. https://doi.org/10.1038/377630a0.
O'Connell J. Fas ligand and the fate of antitumour cytotoxic T lymphocytes. Immunology. 2002;105(3):263–6.
Kang SM, Schneider DB, Lin Z, Hanahan D, Dichek DA, Stock PG, et al. Fas ligand expression in islets of Langerhans does not confer immune privilege and instead targets them for rapid destruction. Nat Med. 1997;3(7):738–43.
Dupont PJ, Warrens AN. Fas ligand exerts its pro-inflammatory effects via neutrophil recruitment but not activation. Immunology. 2007;120(1):133–9. https://doi.org/10.1111/j.1365-2567.2006.02504.x.
Ferguson TA, Griffith TS. A vision of cell death: Fas ligand and immune privilege 10 years later. Immunol Rev. 2006;213:228–38. https://doi.org/10.1111/j.1600-065X.2006.00430.x.
Wu JG, Wilson J, He J, Xiang LB, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Investig. 1996;98(5):1107–13.
Bi LL, Pan G, Atkinson TP, Zheng L, Dale JK, Makris C, et al. Dominant inhibition of Fas ligand-mediated apoptosis due to a heterozygous mutation associated with autoimmune lymphoproliferative syndrome (ALPS) type Ib. BMC Med Genet. 2007;8:41.
Bolze A, Byun M, McDonald D, Morgan NV, Abhyankar A, Premkumar L, et al. Whole-exome-sequencing-based discovery of human FADD deficiency. Am J Hum Genet. 2010;87(6):873–81. https://doi.org/10.1016/j.ajhg.2010.10.028.
Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392(6673):296–300.
Zhu S, Hsu AP, Vacek MM, Zheng L, Schaffer AA, Dale JK, et al. Genetic alterations in caspase-10 may be causative or protective in autoimmune lymphoproliferative syndrome. Hum Genet. 2006;119(3):284–94. https://doi.org/10.1007/s00439-006-0138-9.
Campagnoli MF, Garbarini L, Quarello P, Garelli E, Carando A, Baravalle V, et al. The broad spectrum of autoimmune lymphoproliferative disease: molecular bases, clinical features and long-term follow-up in 31 patients. Haematologica. 2006;91(4):538–41.
Puck JM, Zhu S. Immune disorders caused by defects in the caspase cascade. Curr Allergy Asthma Rep. 2003;3(5):378–84.
Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, et al. Inherited human caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98(1):47–58. https://doi.org/10.1016/S0092-8674(00)80605-4.
Cerutti E, Campagnoli MF, Ferretti M, Garelli E, Crescenzio N, Rosolen A, et al. Co-inherited mutations of Fas and caspase-10 in development of the autoimmune lymphoproliferative syndrome. BMC Immunol. 2007;8:28.
Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419(6905):395–9. https://doi.org/10.1038/nature01063.
Niemela J, Kuehn HS, Kelly C, Zhang M, Davies J, Melendez J, et al. Caspase-8 deficiency presenting as late-onset multi-organ lymphocytic infiltration with granulomas in two adult siblings. J Clin Immunol. 2015;35(4):348–55. https://doi.org/10.1007/s10875-015-0150-8.
Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med. 2005;202(6):727–32. https://doi.org/10.1084/jem.20050683.
Kataoka T, Budd RC, Holler N, Thome M, Martinon F, Irmler M, et al. The caspase-8 inhibitor FLIP promotes activation of NF-kappaB and Erk signaling pathways. Curr Biol. 2000;10(11):640–8.
Shah S, Wu E, Rao VK, Tarrant TK. Autoimmune lymphoproliferative syndrome: an update and review of the literature. Curr Allergy Asthma Rep. 2014;14(9):462. https://doi.org/10.1007/s11882-014-0462-4.
Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome: report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40.
Rensing-Ehl A, Volkl S, Speckmann C, Lorenz MR, Ritter J, Janda A, et al. Abnormally differentiated CD4+ or CD8+ T cells with phenotypic and genetic features of double negative T cells in human Fas deficiency. Blood. 2014;124(6):851–60. https://doi.org/10.1182/blood-2014-03-564286.
Magerus-Chatinet A, Stolzenberg MC, Loffredo MS, Neven B, Schaffner C, Ducrot N, et al. FAS-L, IL-10, and double-negative CD4− CD8− TCR alpha/beta+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113(13):3027–30. https://doi.org/10.1182/blood-2008-09-179630.
Neven B, Bruneau J, Stolzenberg MC, Meyts I, Magerus-Chatinet A, Moens L, et al. Defective anti-polysaccharide response and splenic marginal zone disorganization in ALPS patients. Blood. 2014;124(10):1597–609. https://doi.org/10.1182/blood-2014-02-553834.
van den Berg A, Tamminga R, de Jong D, Maggio E, Kamps W, Poppema S. FAS gene mutation in a case of autoimmune lymphoproliferative syndrome type IA with accumulation of gammadelta+ T cells. Am J Surg Pathol. 2003;27(4):546–53.
Neven B, Magerus-Chatinet A, Florkin B, Gobert D, Lambotte O, De Somer L, et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118(18):4798–807. https://doi.org/10.1182/blood-2011-04-347641.
Rensing-Ehl A, Warnatz K, Fuchs S, Schlesier M, Salzer U, Draeger R, et al. Clinical and immunological overlap between autoimmune lymphoproliferative syndrome and common variable immunodeficiency. Clin Immunol. 2010;137(3):357–65. https://doi.org/10.1016/j.clim.2010.08.008.
Roberts CA, Ayers L, Bateman EA, Sadler R, Magerus-Chatinet A, Rieux-Laucat F, et al. Investigation of common variable immunodeficiency patients and healthy individuals using autoimmune lymphoproliferative syndrome biomarkers. Hum Immunol. 2013;74(12):1531–5. https://doi.org/10.1016/j.humimm.2013.08.266.
Sriram S, Joshi AY, Rodriguez V, Kumar S. Autoimmune lymphoproliferative syndrome: a rare cause of disappearing HDL syndrome. Case Rep Immunol. 2016;2016:7945953. https://doi.org/10.1155/2016/7945953.
Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5(2):182–9. https://doi.org/10.1038/ni1024.
Caminha I, Fleisher TA, Hornung RL, Dale JK, Niemela JE, Price S, et al. Using biomarkers to predict the presence of FAS mutations in patients with features of the autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2010;125(4):946–9 e6. https://doi.org/10.1016/j.jaci.2009.12.983.
Lambotte O, Neven B, Galicier L, Magerus-Chatinet A, Schleinitz N, Hermine O, et al. Diagnosis of autoimmune lymphoproliferative syndrome caused by FAS deficiency in adults. Haematologica. 2013;98(3):389–92. https://doi.org/10.3324/haematol.2012.067488.
Teachey DT, Manno CS, Axsom KM, Andrews T, Choi JK, Greenbaum BH, et al. Unmasking Evans syndrome: T-cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS). Blood. 2005;105(6):2443–8. https://doi.org/10.1182/blood-2004-09-3542.
Lo B, Abdel-Motal UM. Lessons from CTLA-4 deficiency and checkpoint inhibition. Curr Opin Immunol. 2017;49:14–9. https://doi.org/10.1016/j.coi.2017.07.014.
Janda A, Schwarz K, van der Burg M, Vach W, Ijspeert H, Lorenz MR, et al. Disturbed B-lymphocyte selection in autoimmune lymphoproliferative syndrome. Blood. 2016;127(18):2193–202. https://doi.org/10.1182/blood-2015-04-642488.
Boulanger E, Rieux-Laucat F, Picard C, Legall M, Sigaux F, Clauvel JP, et al. Diffuse large B-cell non-Hodgkin’s lymphoma in a patient with autoimmune lymphoproliferative syndrome. Br J Haematol. 2001;113(2):432–4.
Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98(1):194–200.
Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123(13):1989–99. https://doi.org/10.1182/blood-2013-10-535393.
Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118(22):5741–51. https://doi.org/10.1182/blood-2011-07-325217.
Klemann C, Esquivel M, Magerus-Chatinet A, Lorenz MR, Fuchs I, Neveux N, et al. Evolution of disease activity and biomarkers on and off rapamycin in 28 patients with autoimmune lymphoproliferative syndrome. Haematologica. 2017;102(2):e52–e6. https://doi.org/10.3324/haematol.2016.153411.
Sleight BJ, Prasad VS, DeLaat C, Steele P, Ballard E, Arceci RJ, et al. Correction of autoimmune lymphoproliferative syndrome by bone marrow transplantation. Bone Marrow Transplant. 1998;22(4):375–80. https://doi.org/10.1038/sj.bmt.1701306.
Benkerrou M, Le Deist F, de Villartay J, Caillat-Zuckman S, Rieux-Laucat F, Jabado N, et al. Correction of fas (CD95) deficiency by haploidentical bone marrow transplantation. Eur J Immunol. 1997;27:2043–7.
Rieux-Laucat F, Immunology CJL. Autoimmunity by haploinsufficiency. Science. 2014;345(6204):1560–1. https://doi.org/10.1126/science.1260791.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rieux-Laucat, F., Magérus-Chatinet, A. & Neven, B. The Autoimmune Lymphoproliferative Syndrome with Defective FAS or FAS-Ligand Functions. J Clin Immunol 38, 558–568 (2018). https://doi.org/10.1007/s10875-018-0523-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-018-0523-x