Abstract
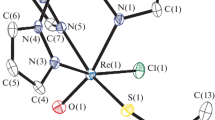
The synthesis, characterization, and reaction mechanism of a series of oxonium derivatives of the rhenium containing metalladecaborane [nido-6-Re(CO)3B9H13][NMe4] are reported herein. The oxonium species were synthesized using tetrahydrofuran, 1,4-dioxane, and diethyl ether. The crystal structure of the tetrahydrofuran derivative [2-(CH2)4O-nido-6-Re(CO)3B9H12] was determined and compared to the isostructural manganese compound. The title compound crystallizes in the Triclinic space group P-1 with (a = 7.0835(5) Å, b = 9.6971(6) Å, c = 12.5762(8) Å, α = 73.5750(10)º, β = 75.5520(10)º, γ = 87.061(2)º, volume = 802.22(9), Z = 2). This facile functionalization strategy will facilitate future investigations of rhenium containing metallaboranes and metallacarboranes.
Graphical Abstract

The synthesis, characterization, and reaction mechanism of a series of oxonium derivatives of the rhenium containing metalladecaborane [nido-6-Re(CO)3B9H13][NMe4] are reported, including the structural determination of [2-(CH2)4O-nido-6-Re(CO)3B9H12].




Similar content being viewed by others
References
Borthakur R, Kar S, Barik SK, Bhattacharya S, Kundu G, Varghese B, Ghosh S (2017) Synthesis, chemistry, and electronic structures of group 9 metallaboranes. Inorg Chem 56:1524–1533. https://doi.org/10.1021/acs.inorgchem.6b02626
Ma P, Littger R, Smith Pellizzeri TM, Zubieta J, Spencer JT (2016) Thermal versus photochemical pathways of a manganese 10-vertex metalladecaborane: [Nido-6-Mn(CO)3B9H13][NMe4]. Polyhedron 109:129–137. https://doi.org/10.1016/j.poly.2016.02.014
Ma P, Littger R, Spencer JT (2018) Thermal and photochemical pathways of a 10-vertex Rhodium metallaborane. Inorg Chim Acta 477:242–247. https://doi.org/10.1016/j.ica.2018.03.009
Ma P, Spencer JT (2018) Photochemistry of metallaborane: a novel method for functionalized carborane synthesis. Inorg Chem Commun 89:78–82. https://doi.org/10.1016/j.inoche.2018.01.009
El-Zaria ME, Janzen N, Valliant JF (2012) Room-temperature synthesis of Re(I) and Tc(I) metallocarboranes. Organometallics 31:5940–5949. https://doi.org/10.1021/om300521j
Goszczyński TM, Kowalski K, Leśnikowski ZJ, Boratyński J (2015) Solid state, thermal synthesis of site-specific protein–boron cluster conjugates and their physicochemical and biochemical properties. Biochim Biophys Acta BBA 1850:411–418. https://doi.org/10.1016/j.bbagen.2014.11.015
Laskova J, Kozlova A, Białek-Pietras M, Studzińska M, Paradowska E, Bregadze V, Leśnikowski ZJ, Semioshkin A (2016) Reactions of closo-dodecaborate amines. Towards novel bis-(closo-dodecaborates) and closo-dodecaborate conjugates with lipids and non-natural nucleosides. J Organomet Chem 807:29–35. https://doi.org/10.1016/j.jorganchem.2016.02.009
Ma P, Spencer JT (2015) Non-covalent stabilization and functionalization of boron nitride nanosheets (BNNSs) by organic polymers: formation of complex BNNSs-containing structures. J Mater Sci 50:313–323. https://doi.org/10.1007/s10853-014-8590-8
Soriano-Ursúa MA, Das BC, Trujillo-Ferrara JG (2014) Boron-containing compounds: chemico-biological properties and expanding medicinal potential in prevention, diagnosis and therapy. Expert Opin Ther Pat 24:485–500. https://doi.org/10.1517/13543776.2014.881472
Toppino A, Genady AR, El-Zaria ME, Reeve J, Mostofian F, Kent J, Valliant JF (2013) High yielding preparation of dicarba-closo-dodecaboranes using a Silver(I) mediated dehydrogenative alkyne-insertion reaction. Inorg Chem 52:8743–8749. https://doi.org/10.1021/ic400928v
Grimes RN (2016) Chap. 2—Structure and bonding. In: Grimes RN (ed) Carboranes, 3rd edn. Academic Press, Boston, pp 7–18
Hosmane NS (2011) Boron science: new technologies and applications, 1st edn. CRC Press, Boca Raton
Ma P, Spencer JT (2018) Cyclodimerization of isocyanates promoted by one large vertex metallaborane. Polyhedron 149:148–152. https://doi.org/10.1016/j.poly.2018.04.028
Ma P, Spencer JT (2018) Catalytic activity of a large Rhodium metallaborane towards the [2 + 2 + 2] cycloaddition of alkynes. Inorg Chim Acta 482:67–69. https://doi.org/10.1016/j.ica.2018.05.041
Bulsink P, Al-Ghamdi A, Joshi P, Korobkov I, Woo T, Richeson D (2016) Capturing Re(I) in an neutral N,N,N pincer Scaffold and resulting enhanced absorption of visible light. Dalton Trans 45:8885–8896. https://doi.org/10.1039/C6DT00661B
Chakraborty I, Jimenez J, Sameera WMC, Kato M, Mascharak PK (2017) Luminescent Re(I) carbonyl complexes as trackable PhotoCORMs for CO delivery to cellular targets. Inorg Chem 56:2863–2873. https://doi.org/10.1021/acs.inorgchem.6b02999
Chakraborty I, Tena J, Mascharak PK (2017) Photoactive rhenium carbonyl complexes of N,N,S-donor ligands: contrast in binding modes based on flexibility of ligand frames and nature of ancillary ligands. Inorg Chim Acta 467:358–363. https://doi.org/10.1016/j.ica.2017.08.015
Chanawanno K, Rhoda HM, Hasheminasab A, Crandall LA, King AJ, Herrick RS, Nemykin VN, Ziegler CJ (2016) Using hydrazine to link ferrocene with Re(CO)3: a modular approach. J Organomet Chem 818:145–153. https://doi.org/10.1016/j.jorganchem.2016.06.004
Hostachy S, Policar C, Delsuc N (2017) Re(I) carbonyl complexes: multimodal platforms for inorganic chemical biology. Coord Chem Rev 351:172–188. https://doi.org/10.1016/j.ccr.2017.05.004
Ismail MB, Booysen IN, Akerman MP (2017) Rhenium(I) complexes with aliphatic Schiff bases appended to bio-active moieties. Inorg Chem Commun 78:78–81. https://doi.org/10.1016/j.inoche.2017.03.007
Jürgens S, Herrmann WA, Kühn FE (2014) Rhenium and technetium based radiopharmaceuticals: development and recent advances. J Organomet Chem 751:83–89. https://doi.org/10.1016/j.jorganchem.2013.07.042
Louie AS, Harrington LE, Valliant JF (2012) The preparation and characterization of functionalized carboranes and Re/Tc-metallocarboranes as platforms for developing molecular imaging probes: structural and cage isomerism studies. Inorg Chim Acta 389:159–167. https://doi.org/10.1016/j.ica.2012.03.017
Goodreau BH, Spencer JT (1992) Small heteroborane cluster systems. 5. Factors affecting the 2D 11B-11B (boron-11) COSY NMR spectra of terminal- and bridge-substituted pentaborane cluster systems. Inorg Chem 31:2612–2621. https://doi.org/10.1021/ic00038a056
James TL, McDonald GG (1973) Measurement of the self-diffusion coefficient of each component in a complex system using pulsed-gradient fourier transform NMR. J Magn Reson 1969 11:58–61. https://doi.org/10.1016/0022-2364(73)90081-4
Levy GC, Peat IR (1975) The experimental approach to accurate carbon-13 spin-lattice relaxation measurements. J Magn Reson 1969 18:500–521. https://doi.org/10.1016/0022-2364(75)90106-7
Shriver DF, Drezdzon MA (1986) The manipulation of air-sensitive compounds, 2nd edn. Wiley, Chichester
Lott JW, Gaines DF (1974) Manganese and rhenium metalloboranes containing tridentate borane ligands tridecahydrononaborate(2-) and (tetrahydrofuran or diethyl ether)dodecahydrononaborate(1-). Inorg Chem 13:2261–2267. https://doi.org/10.1021/ic50139a040
Hope H (1994) Progress in inorganic chemistry, volume 41. Wiley, Chichester
APEX II (2011) APEX II, Data Collection Software. Bruker-AXS Inc., Madison
SAINT plus (2013) SAINT plus, Data Reduction Software. Bruker-AXS Inc., Madison
Sheldrick GM (1996) SADABS. University of Göttingen
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122. https://doi.org/10.1107/S0108767307043930
Ma P, Smith TM, Zubieta J, Spencer JT (2014) Synthesis of alkoxy derivatives of the 10-vertex manganadecaborane [nido-6-Mn(CO)3B9H13][NMe4]. Inorg Chem Commun 46:223–225. https://doi.org/10.1016/j.inoche.2014.04.038
Bernard R, Cornu D, Perrin M, Scharff JP, Miele P (2004) Synthesis and X-ray structural characterisation of the tetramethylene oxonium derivative of the hydrodecaborate anion. A versatile route for derivative chemistry of [B10H10]2–. J Organomet Chem 689:2581–2585. https://doi.org/10.1016/j.jorganchem.2004.05.014
Lesnikowski ZJ (2009) Nucleoside–boron cluster conjugates—beyond pyrimidine nucleosides and carboranes. J Organomet Chem 694:1771–1775. https://doi.org/10.1016/j.jorganchem.2008.12.061
Plešek J, Grüner B, Heřmánek S, Báča J, Mareček V, Jänchenová J, Lhotský A, Holub K, Selucký P, Rais J, Císařová I, Čáslavský J (2002) Synthesis of functionalized cobaltacarboranes based on the closo-[(1,2-C2B9H11)2–3,3′-Co]—ion bearing polydentate ligands for separation of M3+ cations from nuclear waste solutions. Electrochemical and liquid–liquid extraction study of selective transfer of M3+ metal cations to an organic phase. Molecular structure of the closo-[(8-(2-CH3O-C6H4-O)-(CH2CH2O)2—1,2-C2B9H10)-(1′,2′-C2B9H11)-3,3′-Co]Na determined by X-ray diffraction analysis. Polyhedron 21:975–986. https://doi.org/10.1016/S0277-5387(02)00865-3
Sivaev IB, Kulikova NY, Nizhnik EA, Vichuzhanin MV, Starikova ZA, Semioshkin AA, Bregadze VI (2008) Practical synthesis of 1,4-dioxane derivative of the closo-dodecaborate anion and its ring opening with acetylenic alkoxides. J Organomet Chem 693:519–525. https://doi.org/10.1016/j.jorganchem.2007.11.027
Sivaev IB, Starikova ZA, Sjöberg S, Bregadze VI (2002) Synthesis of functional derivatives of the [3,3′-Co(1,2-C2B9H11)2]− anion. J Organomet Chem 649:1–8. https://doi.org/10.1016/S0022-328X(01)01352-3
Zhizhin KY, Mustyatsa VN, Malinina EA, Matveev EY, Goeva LV, Polyakova IN, Kuznetsov NT (2005) Nucleophilic cleavage of cyclic substituents in derivatives of the closo-decaborate anion. Russ J Inorg Chem 50:203209
Semioshkin AA, Sivaev IB, Bregadze VI (2008) Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans 0:977–992. https://doi.org/10.1039/B715363E
Matveev EY, Kubasov AS, Razgonyaeva GA, Polyakova IN, Zhizhin KY, Kuznetsov NT (2015) Reactions of the [B10H10]2–anion with nucleophiles in the presence of halides of group IIIA and IVB elements. Russ J Inorg Chem 60:776–785. https://doi.org/10.1134/S0036023615070104
Kim Y, Deng H, Meek DW, Wojcicki A (2002) Unusual molecular hydrogen complex of rhenium: a long hydrogen-hydrogen bond and inertness to substitution. https://pubs.acs.org/doi/abs/10.1021/ja00163a050. Accessed 10 Oct 2018
Janoušek Z, Plešek J, Heřmánek S, Baše K, Todd LJ, Wright WF (1981) Synthesis and characteristics of sulfur interligand bridge-derivatives and of some S-substituted compounds in the (C2B9H11)2Co– series. Conformations of (C2B9H11)2Mx– metallocarboranes. Collect Czechoslov Chem Commun 46:2818–2833. https://doi.org/10.1135/cccc19812818
Acknowledgements
We wish to thank the Department of Chemistry and the Forensic and National Security Sciences Institute (FNSSI) for their generous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, P., Pellizzeri, T.M.S., Zubieta, J. et al. Synthesis and Characterization of Oxonium Functionalized Rhenium Metallaborane. J Chem Crystallogr 50, 14–20 (2020). https://doi.org/10.1007/s10870-018-0749-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0749-8