Abstract
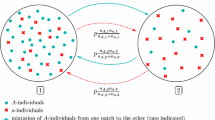
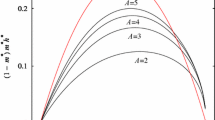
Neutral models of speciation based on isolation by distance and assortative mating, termed topopatric, have shown to be successful in describing abundance distributions and species–area relationships. Previous works have considered this type of process in the context of haploid genomes. Here we discuss the implementation of two schemes of dominance to analyze the effects of diploidy: a complete dominance model in which one allele dominates over the other and a perfect codominant model in which heterozygous genotypes give rise to a third phenotype. In the case of complete dominance, we observe that speciation requires stronger spatial inbreeding in comparison to the haploid model. For perfect codominance, instead, speciation demands stronger genetic assortativeness. Nevertheless, once speciation is established, the three models predict the same abundance distributions even at the quantitative level, revealing the robustness of the original mechanism to describe biodiversity features.





Similar content being viewed by others
References
Coyne J. A., Orr H. A.: Speciation. 1st ed. Sinauer Associates, Inc (2004)
Nosil, P.: Ecological Speciation. Oxford University Press, Oxford (2012)
Gavrilets, S.: Models of speciation: Where are we now? J. Hered. 105(Special Issue), 743–755 (2014)
Hubbell, S.: The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press, Princeton (2001)
Kopp, M.: Speciation and the neutral theory of biodiversity. BioEssays 32, 564–570 (2010)
Etienne, R.S., Haegeman, B.: The neutral theory of biodiversity with random fission speciation. Theor. Ecol. 4, 87–109 (2011)
Rosindell, J., Phillimore, A.B.: A unified model of island biogeography sheds light on the zone of radiation. Ecol. Lett. 14, 552–560 (2011)
Mayr, E.: Animal Species and Evolution. Belknap Press, Cambridge (1963)
Rozenzweig, M.L.: Tempo and mode of speciation. Science 277, 1622–1623 (1997)
Dieckmann, U., Doebeli, M.: On the origin of species by sympatric speciation. Nature 400, 354–357 (1999)
Leimar, O., Doebeli, M., Dieckmann, U.: Evolution of phenotypic clusters through competition and local adaptation along an environmental gradient. Evolution 62(4), 807–822 (2008)
Baptestini, E.M., de Aguiar, M.A.M., Araujo, M.S., Bolnick, D.: The shape of the competition and carrying capacity kernels affects the likelihood of disruptive selection. J. Theor. Biol. 259, 5–11 (2009)
Pinho, C., Hey, J.: Divergence with gene flow: models and data. Annu. Rev. Ecol. Evol. Syst. 41, 215–230 (2010)
de Aguiar, M.A.M., Baranger, M., Baptestini, E.M., Kaufman, L., Bar-Yam, Y.: Global patterns of speciation and diversity. Nature 460, 384–387 (2009)
Hoelzer, G.A., Drewes, R., Meier, J., Doursat, R.: Isolation-by-distance and outbreeding depression are sufficient to drive parapatric speciation in the absence of environmental influences. PLoS Comput. Biol. 4, e1000126 (2008)
Melian, C.J., Alonso, D., Vázquez, D.P., Regetz, J., Allesina, S.: Frequency-dependent selection predicts patterns of radiations and biodiversity. PLoS Comp. Biol. 6, e100089 (2010)
Fuentes, M.A., Kuperman, M.N., Kenkre, V.M.: Nonlocal interaction effects on pattern formation in population dynamics. Phys. Rev. Lett. 91(15), 158104 (2003)
Meyer, M., Havlin, S., Bunde, A.: Clustering of independently diffusing individuals by birth and death processes. Phys. Rev. E 54, 5567–5570 (1996)
Young, W.R., Roberts, A.J., Stuhne, G.: Reproductive pair correlations and the clustering of organisms. Nature 412, 328–331 (2001)
Houchmandzadeh, B.: Clustering of diffusing organisms. Phys. Rev. E 66, 052902 (2002)
Lawson, D.J., Jensen, H.J.: Understanding clustering in type space using field theoretic techniques. Bull. Math. Biol. 70, 1065–1081 (2007)
Irwin, D.E., Bensch, S., Price, T.D.: Speciation in a ring. Nature 409, 333–337 (2001)
Irwin, D.E., Bensch, S., Irwin, J.H., Price, T.D.: Speciation by distance in a ring species. Science 307, 414–416 (2005)
Ashlock, D., Clare, E.L., von Königslöw, T.E., Ashlock, W.: Evolution and instability in ring species complexes: an in silico approach to the study of speciation. J. Theor. Biol. 264, 1202–1213 (2010)
Martins, A.B., de Aguiar, M.A.M., Bar-Yam, Y.: Evolution and stability of ring species. Proc. Natl. Acad. Sci. U.S.A. 110, 5080–5084 (2013)
Scott, A.D., King, D.M., Marić, N., Bahar, S.: Clustering and phase transitions on a neutral landscape. Europhys. Lett. 102(6), 68003 (2013)
Baptestini, E.M., de Aguiar, M.A.M., Bar-Yam, Y.: The role of sex separation in neutral speciation. J. Theor. Ecol. 6, 213–223 (2013)
Wright, S.: Breeding structure of populations in relation to speciation. Am. Nat. 74, 232–248 (1940)
Wright, S.: Isolation by distance. Genetics 28, 114–138 (1943)
Kimura, M., Weiss, G.H.: The stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics 49, 561–576 (1964)
Higgs, P., Derrida, B.: Genetic distance and species formation in evolving populations. J. Mol. Evol. 35, 454–465 (1992)
Dieckmann, U., Doebeli, M.: Evolutionary branching and sympatric speciation caused by different types of ecological interactions. Am. Nat. 156, S77–S101 (2000)
Doebeli, M., Dieckmann, U.: Speciation along environmental gradients. Nature 421, 259–264 (2003)
Bolnick, D.I., Kirkpatrick, M.: The relationship between intraspecific assortative mating and reproductive isolation between divergent populations. Curr. Zool. 58(3), 484–492 (2012)
Parker, G.A., Partridge, L.: Sexual conflict and speciation. Phil. Trans. R. Soc. B 353, 261–274 (1998)
Schneider, D.M., do Carmo, E., Martins, A.B., de Aguiar, M.A.M.: Toward a theory of topopatric speciation: the role of genetic assortative mating. Physica A 409, 35–47 (2014)
Schneider, D.M., do Carmo, E., de Aguiar, M.A.M.: A dynamical analysis of allele frequencies in populations evolving under assortative mating and mutations. Physica A 421, 54–68 (2015)
de Aguiar, M.A.M., Schneider, D.M., do Carmo, E., Campos, P.R.A., Martins, A.B.: Error catastrophe in populations under similarity-essential recombination. J. Theor. Biol. 374, 48–53 (2015)
Eigen, M., Schuster, P.: The Hypercycle. A principle of natural self-organization. Naturwissenschaften 64, 541–565 (1977)
Schneider, D. M., Martins A. B., de Aguiar M. A. M. The mutation-drift balance in spatially structured populations. In preparation
Higgs, P.G., Derrida, B.: Stochastic models for species formation in evolving populations. J. Phys. A 24, L985–L991 (1991)
Gavrilets, S.: Fitness Landscapes and the Origin of Species. Princeton University Press, Princeton (2004)
Mayr E.: In: Arai R., Kato M., Doi Y. (eds) Biodiversity and Evolution. National Science Museum Foundation, Tokyo (1955)
de Aguiar, M.A.M., Bar-Yam, Y.: The Moran model as a dynamical process on networks and its implications for neutral speciation. Phys. Rev. E 84, 031901 (2011)
Acknowledgments
This work was partially supported by CNPq and FAPESP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, D.M., Baptestini, E.M. & de Aguiar, M.A.M. Diploid versus haploid models of neutral speciation. J Biol Phys 42, 235–245 (2016). https://doi.org/10.1007/s10867-015-9404-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10867-015-9404-1