Abstract
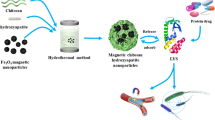
Hydroxyapatite (HAp) and hydroxyapatite/chitosan/β-cyclodextrin (HAp/CS/β-CD) nanoparticles were successfully prepared in the modified simulated body fluid (SBF) solution at the physiological conditions (pH 7.4, temperature = 37 °C). CS/β-CD nanoparticles acted as templates for the synthesis of HAp/CS/β-CD nanoparticles to improve the nanoarchitecture of HAp and its crystallinity.The nanoparticles were characterized by FT-IR spectroscopy, scanning electron microscopy (SEM), and X-ray diffraction (XRD). Kneading and coprecipitation methods were applied to prepare the inclusion complex involving β-CD and p-THPP (5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin), a photosensitizer for anti-cancer drugs. The 1:1 stoichiometric ratio of the formed inclusion complex was characterized by a formation constant of 7.216 × 102 mol−1 dm3 and analyzed by 1H NMR, FTIR, and UV–Vis. The p-THPP delivery release in vitro was in this order: HAp/CS/β-CD < CS/β-CD < < HAp/β-CD < β-CD, hinting at a better controlled release by HAp/CS/β-CD nanoparticles.














Similar content being viewed by others
References
Yuan, Z., Ye, Y., Gao, F., Yuan, H., Lan, M., Lou, K., Wang, W.: Chitosan-graft-β-cyclodextrin nanoparticles as a carrier for controlled drug release. Int. J. Pharm. 446, 191–198 (2013)
Yih, T.C., Al-Fandi, M.: Engineered nanoparticles as precise drug delivery systems. J. Cell. Biochem. 97, 1184–1190 (2006)
Moore, T.L., Schreurs, A.S., Morrison, R.A., Jelen, E.K., Loo, J., Globus, R.K., Alexis, F.: Polymer-coated hydroxyapatite nanoparticles for the delivery of statins. J. Nanomed. Nanotechnol. 5, 237 (2014). https://doi.org/10.4172/2157-7439.1000237
Huang, W., Zhang, J., Dorn, H.C., Zhang, C.: Assembly of bio-nanoparticles for double controlled drug release. PLoS ONE (2013). https://doi.org/10.1371/journal.pone.0074679
Jafari, S., Adibkia, K.: Application of hydroxyapatite nanoparticle in the drug delivery systems. J. Mol. Pharm. Org. Process Res. 3(1), e118 (2015). https://doi.org/10.4172/2329-9053.1000e118
Kaur, S., Bala, N., Khosla, C.: Preparation and deposition of hydroxyapatite on biomaterials by sol-gel technique—a review. Chitkara Chem. Rev. 1, 59–69 (2013)
Salata, O.V.: Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. (2004). https://doi.org/10.1186/1477-3155-2-3
Nayak, A.K.: Hydroxyapatite synthesis methodologies: an overview. Int. J. ChemTech. Res. 2, 903–907 (2011)
Dorozhkin, S.V.: Biocomposites and hybrid biomaterials based on calcium orthophosphates. Biomatter 1, 3–56 (2011). https://doi.org/10.4161/biom.1.1.16782
Brundavanam, S., Eddy, G., Poinern, J., Fawcett, D.: Synthesis of a hydroxyapatite nanopowder via ultrasound irradiation from calcium hydroxide powders for potential biomedical applications. Nanosci. Nanoeng. 3, 1–7 (2015). https://doi.org/10.13189/nn.2015.030101
Manoj, M., Subbiah, R., Mangalaraj, D., Ponpandian, N., Viswanathan, C., Park, K.: Influence of growth parameters on the formation of hydroxyapatite (HAp) nanostructures and their cell viability studies. Nanobiomedicine (2015). https://doi.org/10.5772/60116
Ferraz, M.P., Monteiro, F.J., Manuel, C.M.: Hydroxyapatite nanoparticles: a review of preparation methodologies. J. Appl. Biomater. Biomech. 2, 74–80 (2004)
Foroughi, F., Hassanzadeh-Tabrizi, S.A., Bighamb, A.: In situ microemulsion synthesis of hydroxyapatite-MgFe2O4 nanocomposite as a magnetic drug delivery system. Mater. Sci. Eng. C Mater. Biol. Appl. 68, 774–779 (2016)
Xu, Q., Czernuszka, J.T.: Controlled release of amoxicillin from hydroxyapatite-coated poly(lactic-co-glycolic acid) microspheres. J. Control. Release 127, 146–153 (2008)
Venkatesan, J., Vinodhini, P.A., Sudha, P.N., Kim, S.K.: Chitin and chitosan composites for bone tissue regeneration. Adv. Food Nutr. Res. 73, 59–81 (2014)
Kim, J.-H., Kim, Y.-S., Park, K., Lee, S., Nam, H.-Y., Min, K.-H., Jo, H.-G., Park, J.-H., Choi, K., Jeong, S.-Y., Park, R.-W., Kim, I.-S., Kim, K., Kwon, I.C.: Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release 127, 41–49 (2008)
Yang, S.-J., Shieh, M.-J., Lin, F.-H., Lou, P.-J., Peng, C.-L., Wei, M.-F., Yao, C.-J., Lai, P.-S., Young, T.-H.: Colorectal cancer cell detection by 5-aminolaevulinic acid-loaded chitosan nano-particles. Cancer Lett. 273, 210–220 (2009)
Wu, Y., Yang, W., Wang, C., Hu, J., Fu, S.: Chitosan nanoparticles as a novel delivery system for ammonium glycyrrhizinate. Int. J. Pharm. 295, 235–245 (2005)
El-Kemary, M., Douhal, A.: Photochemistry and photophysics of cyclodextrin caged drugs: Relevance to their stability and efficiency. In: Douhal, A. (ed.) Cyclodextrin materials photochemistry, photophysics and photobiology, Chap. 4, pp. 79–106. Elsevier, London (2006)
Bautista-Sanchez, A., Kasselouri, A., Desroches, M.C., Blais, J., Maillard, P., de Oliveira, D.M., Tedesco, A.C., Prognon, P., Delaire, J.: Photophysical properties of glucoconjugated chlorins and porphyrins and their associations with cyclodextrins. J. Photochem. Photobiol. B 81, 154–162 (2005)
Qiu, W.-G., Li, Z.-F., Bai, G.-M., Meng, S.-N., Dai, H.-X., He, H.: Study on the inclusion behavior between meso-tetrakis[4-(3-pyridiniumpropoxy)phenyl]porphyrin tetrakisbromide and β-cyclodextrin derivatives in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 66, 1189–1193 (2007)
Guo, Y.-J., Chao, J.-B., Pan, J.-H.: Study on the interaction of 5-pyridine-10,15,20-tris-(p-chlorophenyl)porphyrin with cyclodextrins and DNA by spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 68, 231–236 (2007)
Rebiero, A.O., Neri, C.R., Iamamoto, Y., Serra, O.A.: Spectroscopic studies on the inclusion complexes of tetrakis(2-hydroxy-(-nitrophenyl)porphyrin with α-cyclodextrin in solution and in sol-gel matrix. Mater. Sci. 20, 21–27 (2002)
Vinodh, M., Alipour, F.H., Mohamod, A.A., Al-Azemi, T.F.: Molecular assemblies of porphyrins and macrocyclic receptors: Recent developments in their synthesis and applications. Molecules 17, 11763–11799 (2012)
Ricchelli, F.: Photophysical properties of porphyrins in biological membranes. J. Photochem. Photobiol. B29, 109–118 (1995)
Miclea, L.M., Vlaia, L., Vlaia, V., Hădărugă, D.I., Mircioiu, C.: Preparation and characterization of inclusion complexes of meloxicam and α-cyclodextrin and β-cyclodextrin. Farmacia 58, 583–593 (2010)
Ikeda, A., Satake, S., Mae, T., Ueda, M., Sugikawa, K., Shigeto, H., Funabashi, H., Kuroda, A.: Photodynamic activities of porphyrin derivative–cyclodextrincomplexesbyphotoirradiation. ACS Med. Chem. Lett. 8, 555–559 (2017)
Tas, A.C.: Synthesis of biomimetic Ca-hydroxyapatite powders at 37 °C in synthetic body fluids. Biomaterials 21, 1429–1438 (2000)
Heng, C., Zheng, X., Liu, M., Xu, D., Huang, H., Deng, F., Hui, J., Zhang, X., Wei, Y.: Fabrication of luminescent hydroxyapatite nanorods through surface-initiated raft polymerization: characterization, biological imaging and drug delivery applications. Appl. Surf. Sci. 386, 269–275 (2016)
Varadarajan, N., Balu, R., Rana, D., Ramalingam, M., Kumar, T.S.S.: Accelerated sonochemical synthesis of calcium deficient hydroxyapatite nanoparticles. J. Biomater. Tissue Eng. 4, 295–299 (2014)
Yoruç, A.B.H., Koca, Y.: Double step stirring: A novel method for precipitation of nano-sized hydroxyapatite powder. Dig. J. Nanomater. Biostruct. 4, 73–81 (2009)
Cai, Y., Liu, Y., Yan, W., Hu, Q., Tao, J., Zhang, M., Shic, Z., Tang, R.: Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 17, 3780–3787 (2007)
Wikene, K.O., Bruzell, E., Tønnesen, H.H.: Improved antibacterial phototoxicity of a neutral porphyrin in natural deep eutectic solvents. J. Photochem. Photobiol. B. 148, 188–196 (2015)
Kokubo, T., Kushitani, H., Sakka, S., Kitsugi, T., Yamamuro, T.: Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 24, 721–734 (1990)
Kokubo, T.: Surface chemistry of bioactive glass ceramics. J. Non-Cryst. Solids 120, 138–151 (1990)
Kokubo, T., Takadama, H.: How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27, 2907–2915 (2006)
Marques, M.R.C., Löbenberg, R., Almukainzi, M.: Simulated biological fluids with possible application in dissolution testing. Dissolut. Technol. 18, 15–21 (2011)
Bergh, V.J.V., Tønnesen, H.H.: Interactions and solubilization of 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin with poloxamer 407 and β-cyclodextrin-derivatives in binary and ternary systems. J. Drug Delivery Sci. Technol. 37, 51–60 (2017)
Harada, A., Takahashi, S.: Preparation and properties of inclusion complexes of 1, 2-dicarbadodecaborane (12) with cyclodextrins. J. Chem. Soc. Chem. Commun. 20, 1352–1353 (1988)
Xiao, X., Liu, R., Qiu, C., Zhu, D., Liu, F.: Biomimetic synthesis of micrometer spherical hydroxyapatite with β-cyclodextrin as template. Mater. Sci. Eng. C Mater. Biol. Appl. 29, 785–790 (2009)
Chou, Y.-F., Chiou, W.-A., Xu, Y., Dunn, J.C.Y., Wu, B.M.: The effect of pH on the structural evolution of accelerated biomimetic apatite. Biomaterials 25, 5323–5331 (2004)
Calvo, P., Remuñán-López, C., Vila-Jato, J.L., Alonso, M.J.: Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 63, 125–132 (1997)
Trapani, A., Garcia-Fuentes, M., Alonso, M.J.: Novel drug nanocarriers combining hydrophilic cyclodextrins and chitosan. Nanotechnology 19, 185101 (2008)
Krauland, A.H., Alonso, M.J.: Chitosan/cyclodextrin nanoparticles as macromolecular drug delivery system. Int. J. Pharm. 340, 134–142 (2007)
Roik, N.V., Belyakova, L.A.: IR spectroscopy, X-ray diffraction and thermal analysis studies of solid “β-cyclodextrin-para-aminobenzoic acid” inclusion complex. Phys. Chem. Solid State 12, 168–173 (2011)
Silva, S.M.L., Braga, C.R.C., Fook, M.V.L., Raposo, C.M.O., Carvalho, L.H., Canedo, E.L.: Application of infrared spectroscopy to analysis of chitosan/clay nanocomposites. In: Theophile, T. (ed.) Infrared spectroscopy-materials science, engineering and technology, Chap. 2, pp. 43–62. InTech, Rijeka (2012)
Park, K.H., Kim, S.J., Hwang, M.J., Song, H.J., Park, Y.J.: Biomimetic fabrication of calcium phosphate/chitosan nanohybrid composite in modified simulated body fluids. Express Polym. Lett. 11, 14–20 (2017)
Arsad, M.S.M., Lee, P.M., Hung, L.K.: Synthesis and characterization of hydroxyapatite nanoparticles and β-TCP particles. 2nd international conference on biotechnology and food science. IPCBEE, Vol. 7, IACSIT Press, Singapore (2011)
Paz, A., Guadarrama, D., López, M., González, J.E., Brizuela, N., Aragón, J.: A comparative study of hydroxyapatite nanoparticles synthesized by different routes. Quim. Nova 35, 1724–1727 (2012)
Arsad, M.S.M., Lee, P.M., Hung, L.K.: Morphology and particle size analysis of hydroxyapatite micro- and nano-particles. International conference on science and social research (CSSR 2010), Kuala Lumpur, Malaysia (2010)
Chandrasekar, A., Sagadevan, S., Dakshnamoorthy, A.: Synthesis and characterization of nano-hydroxyapatite (n-HAP) using the wet chemical technique. Int. J. Phys. Sci. 8, 1639–1645 (2013)
Namasivayam, S.K.R., Kumar, P., Bharani, R.S.A., Nishanth, A.N., Nivedh, S.K.: Cyclodextrin nanoparticles incorporated fluconazole and medicinal plant extracts preparation for the improved anti-fungal activity against human pathogenic fungi. Int. J. Pharm. Tech. Res. 6, 1756–1761 (2014)
Chander, S., Fuerstenau, D.W.: On the dissolution and interfacial properties of hydroxyapatite. Colloids Surf. 4, 101–120 (1982)
Pearce, E.I.F.: On the dissolution of hydroxyapatite in acid solutions. J. Dent. Res. 67, 1056–1058 (1988)
Tung, M.S.: Calcium phosphates: structure, composition, solubility, and stability. In: Amjad, Z. (ed.) Calcium phosphates in biological and industrial systems, pp. 1–19. Kluwer Academic Publishers, Boston (1998)
Ozdemir, F., Evans, I., Bretcanu, O.: Calcium phosphate cements for medical applications. In: Gurbinder Kaur, G. (ed.) Clinical applications of biomaterials: state-of-the-art progress, trends, and novel approaches, pp. 91–121. Springer, Cham (2017)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mzyène, F., Moulay, S., Bal, K. et al. Biomimetic approach towards the preparation of hydroxyapatite and hydroxyapatite/chitosan/β-cyclodextrin nanoparticles: application to controlled drug release. J Incl Phenom Macrocycl Chem 92, 381–394 (2018). https://doi.org/10.1007/s10847-018-0842-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-018-0842-9