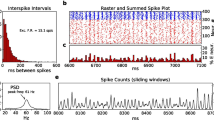
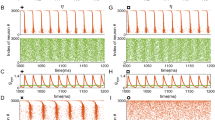
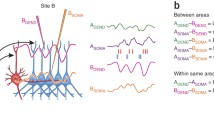
Abstract
Gamma-band synchronization has been linked to attention and communication between brain regions, yet the underlying dynamical mechanisms are still unclear. How does the timing and amplitude of inputs to cells that generate an endogenously noisy gamma rhythm affect the network activity and rhythm? How does such ”communication through coherence” (CTC) survive in the face of rhythm and input variability? We present a stochastic modelling approach to this question that yields a very fast computation of the effectiveness of inputs to cells involved in gamma rhythms. Our work is partly motivated by recent optogenetic experiments (Cardin et al. Nature, 459(7247), 663–667 2009) that tested the gamma phase-dependence of network responses by first stabilizing the rhythm with periodic light pulses to the interneurons (I). Our computationally efficient model E-I network of stochastic two-state neurons exhibits finite-size fluctuations. Using the Hilbert transform and Kuramoto index, we study how the stochastic phase of its gamma rhythm is entrained by external pulses. We then compute how this rhythmic inhibition controls the effectiveness of external input onto pyramidal (E) cells, and how variability shapes the window of firing opportunity. For transferring the time variations of an external input to the E cells, we find a tradeoff between the phase selectivity and depth of rate modulation. We also show that the CTC is sensitive to the jitter in the arrival times of spikes to the E cells, and to the degree of I-cell entrainment. We further find that CTC can occur even if the underlying deterministic system does not oscillate; quasicycle-type rhythms induced by the finite-size noise retain the basic CTC properties. Finally a resonance analysis confirms the relative importance of the I cell pacing for rhythm generation. Analysis of whole network behaviour, including computations of synchrony, phase and shifts in excitatory-inhibitory balance, can be further sped up by orders of magnitude using two coupled stochastic differential equations, one for each population. Our work thus yields a fast tool to numerically and analytically investigate CTC in a noisy context. It shows that CTC can be quite vulnerable to rhythm and input variability, which both decrease phase preference.















Similar content being viewed by others
References
Akam, T., & Kullmann, D.M. (2014). Oscillatory multiplexing of population codes for selective communication in the mammalian brain. Nature Reviews Neuroscience, 14, 111–122.
Bartos, M., Vida, I., & Jonas, P. (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nature Reviews Neuroscience, 8, 45–56.
Benayoun, M., Cowan, J.D., van Drongelen, W., & Wallace, E. (2010). Avalanches in a stochastic model of spiking neurons. Plos Computational Biology, 6(6), e1000,846.
Boashash, B. (1992). Estimating and interpreting the instantaneous frequency of a signal. ii. algorithms and applications. Proceedings of the IEEE, 80(4), 540–568.
Börgers, C., & Kopell, N. (2008). Gamma oscillations and stimulus selection. Neural Computation, 20(2), 383–414.
Bressloff, P.C. (2009). Stochastic neural field theory and the system-size expansion. SIAM Journal of Appllied Mathematics, 70(5), 1488–1521.
Bressloff, P.C. (2010). Metastable states and quasicycles in a stochastic wilson-cowan model of neural population dynamics. Physical Review E, 82(5), 051,903.
Bressloff, P.C., & Newby, J.M. (2013). Metastability in a stochastic neural network modeled as a velocity jump markov process. Preprint (submitted).
Brosch, M., Budinger, E., & Scheich, H. (2002). Stimulus-related gamma oscillations in primate auditory cortex. Journal Neurophysiology, 87(6), 2715–25.
Brunel, N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. Journal of Computational Neuroscience, 8, 183–208.
Brunel, N., & Hakim, V. (1999). Fast global oscillations in networks of integrate-and-fire neurons with low firing rates. Neural Computation, 11(7), 1621–1671.
Buehlmann, A., & Deco, G. (2010). Optimal information transfer in the cortex through synchronization. Plos Computational Biology, 6(9), e1000,934.
Buice, M.A., & Chow, C.C. (2013). Dynamic finite size effects in spiking neural networks. Plos Computational Biology, 9(1), e1002,872.
Buice, M.A., & Cowan, J.D. (2007). Field-theoretic approach to fluctuation effects in neural networks. Physical Review E, 75(5), 051,919.
Buzsàki, G. (2006). Rhythms of the Brain: Oxford University Press.
Buzsáki, G., & Wang, X.J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35(10), 203–225.
Cannon, J., McCarthy, M., Lee, S.J., Börgers, C., Whittington, M., & Kopell, N. (2014). Neurosystems: brain rhythms and cognitive processing. European Journal of Neuroscience, 39(5), 705–719.
Cardin, J., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., Tsai, L., & Moore, C. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459(7247), 663–7.
Csicsvari, J., Jamieson, B., Wise, K., & Buzsáki, G. (2003). Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron, 37(2), 311–22.
Dumont, G., Northoff, G., & Longtin, A. (2014). Linear noise approximation for oscillations in a stochastic inhibitory network with delay. Physical Review E, 90(1).
Rolls, E.T., Webb, T.J., & Deco, G. (2012). Communication before coherence. European Journal of Neuroscience, 36, 2689–2709.
Engel, A.K., Kreiter, A.K., König, P., & Singer, W. (1991). Synchronization of oscillatory neuronal responses between striate and extrastriate visual cortical areas of the cat. Proceedings of the National Academy of Sciences of the United States of America, 88(14), 6048–6052.
Ermentrout, B., & Terman, D. (2010). Mathematical foundations of neuroscience: Springer.
Fries, P. (2005). A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cognitive Science, 9(10), 474–80.
Fries, P., Nikolić, D., & Singer, W. (2007). The gamma cycle. Trends Neuroscience, 30(7), 309–16.
Fries, P., Reynolds, J., Rorie, A., & Desimone, R. (2001). Modulation of oscillatory neuronal synchronization by selective visual attention. Science, 291(5508), 1560–3.
Gillespie, D.T. (2001). Approximate accelerated stochastic simulation of chemically reacting systems. Journal of Chemical Physics, 115(4), 1716–36.
Gillespie, D.T. (2007). Stochastic simulation of chemical kinetics. Annual Review of Physical Chemistry, 58, 35–55.
Ginzburg, I., & Sompolinsky, H. (1994). Theory of correlations in stochastic neural networks. Physical Review E, 50(4), 3171–3191.
Greenwood, P.E., McDonnell, M.D., & Ward, L.M. (2015). Dynamics of gamma bursts in local field potentials. Neural Computation, 27(1), 74–103.
Grytskyy, D., Diesmann, T.T.M., & Helias, M. (2013). A unified view on weakly correlated recurrent networks. Frontiers Computational Neuroscience, 7, 131.
Helias, M., Tetzlaff, T., & Diesmann, M. (2014). The correlation structure of local neuronal networks intrinsically results from recurrent dynamics. Plos Computational Biology, 10(1), e1003,428.
Knoblich, U., Siegle, J.H., Pritchett, D.L., & Moore, C.I. (2010). What do we gain from gamma? local dynamic gain modulation drives enhanced efficacy and efficiency of signal transmission. Front Hum Neurosci, 21(4).
Kukjin Kang, M.S., Henrie, J.A., & Shapley, R. (2010). Lfp spectral peaks in v1 cortex: network resonance and cortico-cortical feedback. Journal of Computational Neuroscience, 29(3), 495–507.
Lachaux, J., Rodriguez, E., Martinerie, J., & Varela, F. (1999). Measuring phase synchrony in brain signals. Human Brain Mapping, 8(4), 194–208.
Lindner, B., Doiron, B., & Longtin, A. (2005). Theory of oscillatory firing induced by spatially correlated noise and delayed inhibitory feedback. Physical Review E, 72(2), 061,919–061,933.
Mazzoni, A., Panzeri, S., Logothetis, N., & Brunel, N. (2008). Encoding of naturalistic stimuli by local field potential spectra in networks of excitatory and inhibitory neurons. Plos Computational Biology, 4, e1000,239.
Northoff, G. (2014). Unlocking the Brain, Volume 1: Coding: Oxford University Press.
Renart, A, de la Rocha, J., Bartho, P., Hollender, L., Parga, N., Reyes, A., & Harris, K.D. (2010). The asynchronous state in cortical circuits. Science, 327(5955), 587–590.
Salinas, E., & Sejnowski, T. (2001). Correlated neuronal activity and the flow of neural information. Nature Reviews Neuroscience, 2(8), 539–50.
Schoffelen, J., Oostenveld, R., & Fries, P. (2005). Neuronal coherence as a mechanism of effective corticospinal interaction. Science, 308(5718), 111–3.
Sejnowski, T., & Paulsen, O. (2006). Network oscillations: emerging computational principles. Journal of Neuroscience, 26(6), 1673–6.
Stopfer, M., Bhagavan, S., Smith, B., & Laurent, G. (1997). Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature, 390(6655), 70–4.
Tiesinga, P. (2012). Motifs in health and disease: the promise of circuit interrogation by optogenetics. European Journal of Neuroscience, 36(2), 2260–72.
Tiesinga, P., & Sejnowski, T. (2009). Cortical enlightenment: are attentional gamma oscillations driven by ing or ping? Neuron, 63, 727–732.
Tiesinga, P.H., & Sejnowski, T.J. (2010). Mechanisms for phase shifting in cortical networks and their role in communication through coherence. Front Hum Neurosci, 4(196).
Tiesinga, P.H.E. (2012). Motifs in health and disease: the promise of circuit interrogation by optogenetics. European Journal of Neuroscience, 36(2).
Wallace, E. (2010). A simplified derivation of the linear noise approximation. arXiv:1004.4280.
Wallace, E., Benayoun, M., van Drongelen, W., & Cowan, J.D. (2011). Emergent oscillations in networks of stochastic spiking neurons. Plos one, 6(5), e14,804.
Wallace, E., Gillespie, D.T., Sanft, K.R., & Petzold, L.R. (2013). A new perspective on the linear noise approximation. Preprint (submitted).
Wang, X., & Buzsáki, G. (1996). Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. Journal of Neuroscience, 16(20), 6402–13.
Wilson, H.R., & Cowan, J.D. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal, 12(1), 1–24.
Womelsdorf, T., Schoffelen, J., Oostenveld, R., Singer, W., Desimone, R., Engel, A., & Fries, P. (2007). Modulation of neuronal interactions through neuronal synchronization. Science, 316(5831), 1609–12.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (AL) and the University of Ottawa Research Chair in Neurophysics (AL). G.N. thanks CIHR (Canadian Institute of Health Research), the Michael Smith Foundations EJLB-Michael Smith Chair program, and the Hope for Depression Research Foundation (HDRF) for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Frances K. Skinner
Rights and permissions
About this article
Cite this article
Dumont, G., Northoff, G. & Longtin, A. A stochastic model of input effectiveness during irregular gamma rhythms. J Comput Neurosci 40, 85–101 (2016). https://doi.org/10.1007/s10827-015-0583-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-015-0583-3